A Technical Note on 2. CO, Ph and CN Vibrational Frequencies in Compounds of Unusual Bonding
Abraham F. Jalbout1,2,3*, Danny F. Jalbout3, Suhong Zhang2 and Tao Jiang2
1Department of Physics,
Dillard University,
New Orleans,
LA 70112
2Department of Chemistry,
University of New Orleans,
New Orleans, LA 70148-2820
3EJMAPS Organization,
1107 Carrollton Ave,
Metairie, LA 70005
* Corresponding Author:Ajalbout [at] ejmaps.org
In our previous technical note on OH and CO frequencies [1] we reported a simple comparison of the stretching frequencies between glyoxalic acid, and calcium acetate. Presently, we report the CN, Ph (phenyl), and CO vibrational frequencies in a variety of organic, inorganic, and organometallic complexes, monomers and polymeric species of broad interest in materials science, chemistry, and industry.
The IR spectra were collected from a Bruker HR120 Fourier transform IR spectrophotometer, and all samples were prepared (from commercial samples when available). For the Re containing clusters we have adopted all of our synthesis techniques from our other sources [2-4], and for the formation of the polymers we reference our recently published work on those systems [5-7].
From Table 1 we can see many vibrational frequencies for CO (alkyl carbonyl groups) containing molecules. For the first molecular complex which is Allantoin (Ca ((OH)2CHCOO)2), we obtained frequencies at 3160, 1610, 1405, 1327, 1283, 1090, 1072, 855, 804 cm-1 .
This led us to believe that the structure is (Figure 1 based on our previous work [1], [9]):
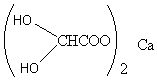
Figure 1. The Proposed Structure of Allantoin
Also, in the table we have listed the CO (alkyl carbonyl groups) frequencies for methyl methacrylate and poly methyl methacrylate and as we can see they are slightly higher than in allantoin, due to the geometrical differences in the structures.
For the CO (carbonyl group) vibrational frequencies of inorganic species, it is often useful to go over a few fundamentals. The typical frequencies for free CO is around 2143 cm-1, whereas terminal CO lies at around 1850-2120 cm-1. Complexes with a symmetrical m2-CO (where M=metal) group (![]() ) have frequencies that generally range between 1700-1860 cm-1 and for symmetrical m3-CO (where M=metal) groups (
) have frequencies that generally range between 1700-1860 cm-1 and for symmetrical m3-CO (where M=metal) groups (![]() ) the frequencies are around 1600-1700 cm-1. This can be better illustrated by the following figure that contains both types of groups:
) the frequencies are around 1600-1700 cm-1. This can be better illustrated by the following figure that contains both types of groups:
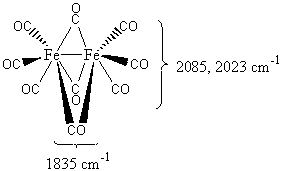
Figure 2. Fe complex with free CO and m2-CO groups
Thus, these trends can be realized in Table 1. To further study the effects of charge on CO frequencies we recorded the IR spectra of three isoelectronic hexacarbonyl complexes ([V (CO) 6]–, [Mn (CO) 6]+, and Cr (CO) 6]. Since V (Vanadium) has a very small nuclear charge it has the weakest ability to attract electrons and also has a tendency to “back” donate electron density to CO. In addition, as it is well known the more negative charge on the species the greater the tendency of metals to donate electrons to the antibonding orbitals of CO, and lower the value of the CO vibrations [9]. Table 1 also shows the CO frequencies in several combinations of Mo containing compounds, which may be of potential use in enzymes (i.e. Mo containing enzymes such as chicken liver Sulfite Oxidase [10]).
| Species | uCO (cm-1) |
| Allantoin | 1610 |
| Methyl methacrylate | 1742 |
| Polymethyl methacrylate | 1739 |
| [V (CO) 6]– | 1860 |
| [Mn (CO) 6]+ | 2099 |
| Cr (CO) 6 | 2000 |
| Fac-Mo (CO) 3 (PF3) 3 | 2091,2058 |
| Fac-Mo (CO) 3 (PCl3) 3 | 2035,1990 |
| Fac-Mo (CO) 3 (PClPH2) 3 | 1980,1887 |
| Fac-Mo (CO) 3 (PMe3) 3 | 1947,1853 |
Table 1. Vibrational frequencies for the CO group (in cm-1)
for several species obtained by Fourier Transform Infrared
Resonance Spectroscopy
From Table 2 the CN vibrational frequencies for several species are presented. As we can see CN in the polymer is quite different to that of the monomer (versus the similarities in the frequencies shifts observed in methyl methacrylate in Table 1, as well as styrene shown in Table 3). We also have shown the vibrational CN modes for Re complexes that contain CN (attached to a methyl group).
| Species | uCN (cm-1) |
| Acrylonitrile | 1921 |
| Polyacrylonitrile | 2240 |
| Cis-[Re6Se8(PEt3)4(MeCN)2](BF4)2 | 2285,2320 |
| [Re6Se8(PEt3)5(MeCN)](BF4)2 | 2278,2384 |
| [Re6Se8(MeCN)6](BF4)2 | 2290,2321 |
Table 2. Vibrational frequencies for the CN group (in cm-1)
for several species obtained by Fourier Transform Infrared
Resonance Spectroscopy
| Species | uPh (cm-1) |
| Styrene | 701 |
| Polystyrene | 715 |
Table 3. Vibrational frequencies for the Ph group (in cm-1)
for several species obtained by Fourier Transform Infrared
Resonance Spectroscopy
We feel that this piece of work might of interest to many researchers in both pure and applied sciences, and should be of use for routine laboratory studies involving inorganic and organic compounds with CO, CN, and Ph groups.
References
- Jalbout, A.F., Zhang, S., Int. J. Vib. Spec., [www.irdg.org/ijvs] 6, 2, 7 (2002)
- Zheng, Z., Long, J.R., Holm, R.H., J. Am. Chem. Soc., 119(9), 2163 (1997)
- Jalbout, A.F., Zheng, Z. (unpublished work)
- Jalbout, A.F., Int. J. Math. Math. Sci. (to appear)
- Jalbout, A.F., Jiang, T., Fengqi, L., Ding, C., Darwish, A.M., Spectrochim. Acta Part A, 58, 525 (2002)
- Jalbout, A.F., Jiang, T., Spectrochim. Acta Part A, 000-000, (2002) (glass transitions)
- Jalbout, A.F., Jiang, T., Spectrochim. Acta Part A, 000-000, (2002) (TEM spectra)
- Jalbout, A.F., Zhang, S., Darwish, A.M., J. Electrochem. Soc. (to be published)
- Shriver, D., Atkins, P., Inorganic Chemistry, 3rd Edition, W.H. Freeman and Compang, New York, Chapter 15 (1999).
- Stiefel, E. I. Proc. Natl. Acad. Sci. U.S.A., 70, 988 (1973); Pacheco, A.; Hazzard, J. T.; Tollin, G.; Enemark, J. H., J. Biol. Inorg. Chem , 4, 390 (1999)
Received 7th May 2002, accepted 7th May 2002.
REF: A.F. Jalbout & S. Zhang Int. J. Vib. Spect., [www.irdg.org/ijvs] 6, 3, 5 (2002)