SERRS – a sensitive spectroscopic technique
68. SERRS – a sensitive spectroscopic technique
Suzanne D. Cooper, W. Ewen Smith*, Caroline Rodger and Peter C. White
Department of Pure and Applied Chemistry, University of Strathclyde, Glasgow, G1 1XL, UK
*to whom correspondence should be addressed
Introduction
69. In theory, surface enhanced resonance Raman scattering (SERRS) is capable of the detection of much less than a monolayer of organic material present on a metal surface. Where the SERRS effect can be applied it has several major advantages over the techniques of surface enhance Raman scattering (SERS) and resonance Raman scattering [1-3]:
- It is effective in both air, water and some solvents as well as in vacuum.
- It provides vibrational information on an adsorbate and it is possible to obtain electronic information through excitation profiles.
- Very wide concentration ranges can be studied using SERRS in contrast to resonance Raman. Detection limits orders of magnitude below monolayer coverage can be obtained in some circumstances; indeed SERRS can have a sensitivity comparable to or better than fluorescence.
- The phenomenon of fluorescence quenching in SERRS means that many more molecules can be studied than with resonance Raman.
- Photolysis is inhibited with SERRS due to the low laser powers necessary to obtain good spectra. In addition, even at high powers the energy transfer between molecule and metal helps prevent photodecomposition.
- The sharp spectral bands obtained enable discrimination of individual molecular species in a mixture. Mixtures of up to four or five chromophores can be discriminated by eye.
- Depending on the conditions used, the technique can be arranged either to detect quantitatively at very low concentrations, or to obtain information on molecular orientation.
70. However the technique has two significant limitations in that:
- the molecule to be studied must contain an appropriate chromophore.
- the chromophore must be in close proximity to a suitably roughened surface of certain metals; in particular silver, copper and gold.
In addition, in practice the method can be used semi-quantitatively in some cases, but many workers have experienced significant difficulty in obtaining reproducible results. This short article explains the nature of the effect and postulates that the lack of reproducibility is due to shortcomings in the understanding and control of the chemistry used.
71. Nature of method
Although SERRS is best considered as a single process it is often thought of as a combination of surface enhanced Raman scattering (SERS) and resonance Raman scattering.
SERS was discovered experimentally some years ago and basically involves the interaction between the molecule adsorbed on the surface and the plasmons present on the surface of certain metals [4]. This greatly enhances the Raman scattering cross section of the molecules, but for this to occur the surface must be roughened. Although the mechanisms whereby surface enhancement is obtained are still the subject of controversy, the nature of the effect is well established and enhancements of Raman band intensities in the range 103 to 106 are often quoted.
72. There have been various attempts to explain the process. Most commonly it is believed that two processes can occur, namely electromagnetic and charge transfer enhancement. In the electromagnetic mechanism the electric field on a rough surface becomes greatly enhanced if the incident photon energy is in resonance with a normal mode of the free electrons in the metal. Where the size of the roughness feature is much less than the wavelength of the incident light it is the dielectric constant of the metal that determines the electric field enhancement. Only certain metals such as silver, gold and copper produce maximum values of the electric field in the visible wavelength region and thus give a large SERS enhancement. A molecule on a rough surface of one of these metals will experience an enhanced electric field from the metal in addition to the electric field from the incident light, thus will scatter light with an enhanced intensity compared to an isolated molecule. This contribution to SERS does not require direct bonding between the adsorbate and the metal surface; enhanced sensitivity has been recorded up to 40 Å from the metal surface. In the charge transfer mechanism, the enhancement effect is dependent on the nature of the molecules involved. It involves transfer of charge from the metal surface through a bond. The enhancement mechanism requires new electronic transitions between adsorbate and metal, made possible when a molecule is at the surface. This mechanism accounts for the high specificity of SERS to the first layer of adsorbed molecules.
73. Resonance Raman scattering occurs when the energy of the incident photons matches (or nearly matches) the energy of an electronic transition in a molecule [5]. This process enhances the efficiency of the scattering process by about a factor of three or four orders of magnitude compared to ordinary Raman scattering.
Electronic information as well as vibrational information can be obtained from the frequency dependence of the intensity of the scattering. In practice, resonance Raman scattering is limited to only certain molecules with chromophores, due to problems with fluorescence, photodecomposition, and self-absorption.
Most SERRS spectra are interpreted on the basis that the resonance and SERS effects can be treated separately. This method is practical where the combination is between resonance Raman and the electromagnetic SERS term. If the charge transfer effect and resonance make the greatest contribution, the chromophore and the charge transfer involved in SERS are likely to be coupled.
74. The substrates used for SERRS can be created in several ways, but the most common are: electrochemical (oxidation and reduction of metal electrodes), evaporation (vacuum deposition of metallic films on a substrate) and chemical reduction (metal colloids formed in solution). Electrode systems for SERRS have the advantage that the surface roughness is easily created and regenerated, and electron transfer and voltage dependence can be easily studied. The problem is that the nature of the surface is difficult to characterise. In the technique extensively studied at Strathclyde, aggregated colloid (usually silver) is used to provide the roughened surface.
75. Experimental Design
SERRS is obtained by using a molecule with a chromophore as the adsorbate and selecting the excitation frequency to match that of the chromophore as shown in figure 1.
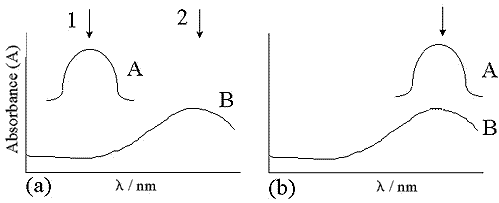
Figure 1: The different arrangements for SERRS: A, molecular absorbance; B, plasmon absorbance. In (a) the molecular absorbance maximum and the plasmon absorbance do not coincide. The two positions 1 and 2 represent excitation at the molecular absorbance maximum and the plasmon absorbance maximum respectively.
If the excitation is at molecular resonance (position 1 in figure 1(a)), the interaction with the chromophore partially depolarises the incident light beam which reduces, but may not eliminate, the dependence of the signal on the orientation of the adsorbate on the surface. This makes the technique less sensitive to small changes in experimental conditions, and is the ideal condition for quantitative detection at attomolar levels. If the excitation is off-resonance for the molecular chromophore but at resonance for the surface plasmons (position 2 in figure 1(a)), the condition is sometimes known as SE(R)RS. Pre-resonance still gives selectivity or sensitivity for the species containing the chromophore, but the scattering intensities are dependent on the molecular orientation at the surface, providing more information on the nature of the bonding.
76. Figure 1(b) illustrates an alternative situation where the molecular absorbance and plasmon resonance maxima coincide. The enhancement will be similar to the first case described for figure 1(a) but the enhancement factor is likely to be greater.
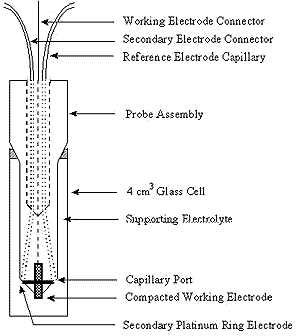
Figure 2: Design for electrochemical cell
Many types of surfaces have been used for SERRS. The most traditional surface and one of the best is a roughened silver electrode. This is achieved by carrying out an electrochemical step prior to the adsorption of the molecules onto the substrate. Figure 2 shows a possible electrode system. The advantages of a system of this type is that it can be incorporated into a 1 cm fluorescence cell making sampling relatively simple. However, the flexibility of sampling arrangements in Raman are one of its big advantages, and consequently many cell types can be used. In particular, the correct positioning of the secondary electrode can be better achieved by other designs of cell.
77. Many other SERS active surfaces have been suggested. Some examples are cold deposition of silver island films on surfaces and ruled gratings. Lately, the use of aggregated silver colloids has become quite widespread. These aggregated colloids provide very good roughened surfaces with high electric fields in the interstices, and consequently provide a very effective scattering surface. Two particular colloid types are most commonly used. In one, borohydride reduction is used to obtain the colloid. The alternative is to use a citrate reduction process. In this laboratory, the citrate reduction process is preferred since the resultant colloids have a long lifetime. A sample of colloid can be used for periods of up to 6 months following preparation. This particular colloid is rather more difficult to make but if made successfully can be more reproducible than the borohydride colloid.
Silver colloids are prepared at Strathclyde using a modified Lee and Meisel procedure [6]. Before use all glassware is cleaned using aqua regia, followed by gentle scrubbing with soap solution and is then successively rinsed in distilled water and methanol. 500 ml of distilled water and 90 mg of silver nitrate are added to a round bottomed flask. The flask is then heated to boiling with vigorous stirring, then 10 ml of a 1% solution of trisodium citrate is added. The heating is then reduced and the solution is left to boil gently for 90 minutes with continuous stirring. The flask is then left to cool and the volume adjusted to 500 ml with addition of distilled water. The quality of the colloid produced is analysed using a uv-vis spectrometer; the λmax should be approximately 404 nm and the full width at half height less than 60 nm. The advantage of the method is that the colloid is reproducible and if produced in this manner can be stable for several months.
78. Characterisation of a standard SERRS colloid
The surface of the citrate-reduced silver colloid has been investigated and found to consist of a layer of citrate with pendant negatively charged groups [7]. Positively charged molecules such as Rhodamine 6G therefore readily adhere to the surface and thus give good SERRS spectra, but negatively charged molecules and some neutral species do not.
To obtain a rough surface suitable for SERRS, the colloid must first be aggregated. A fractal cluster is then obtained where the electric field intensity is very high in the interstices of the aggregates; an enhancement of 106 is expected for SERS with this method. Aggregation can be performed in several ways by the addition of a suitable aggregating agent chosen to be appropriate to the adsorbate studied.
79. A critical feature required to obtain reproducible spectra on colloid is that the aggregation step must be carefully controlled. The most standard aggregating agents are either inorganic salts such as sodium nitrate or acid solutions. More recently, other methods using poly-L-lysine to aggregate the colloid have been suggested. The advantages of these methods are that the poly-L-lysine contains positive charges which can attract negative species to the negative colloid particle surfaces, and also poly-L-lysine helps to act as an aggregation control agent. It is essential that stable aggregates are obtained for a period of time significantly longer than that required to take the spectrum.
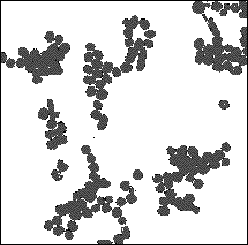
Figure 3: TEM photograph of poly-L-lysine aggregated colloid
Transmission and scanning electron microscopy were used to characterise the colloid particles; the particles were found to hexagonal in shape with a largest face dimension of 36 nm. Aggregated colloid was also analysed; figure 3 shows a TEM photograph of poly-L-lysine aggregated silver colloid. Colloid aggregated with poly-L-lysine consists of small clumps of particles, but physical contact between them is prevented by a layer of poly-L-lysine on each particle. Conversely acid aggregated colloid consists of clumps of particles but with no space between the particles; as the amount of acid added is increased the size of these aggregates also increases.
80. SERRS Applications
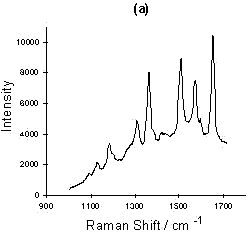
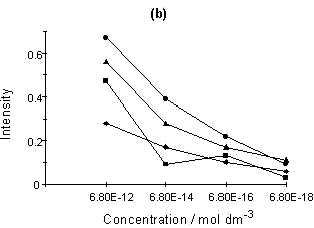
Figure 4: (a) SERRS spectrum of Rhodamine 6G; (b) Relationship of intensity vs. concentration for four peaks selected from the Rhodamine 6G SERRS spectra using acid aggregation:
■, 612; ▲, 1510; ◆, 1578; ●, 1650 cm-1
The sensitivity of the SERRS technique is now well understood. A number of papers claiming very sensitive detection and indeed single molecular detection are appearing [8, 9]. An example of this is shown for Rhodamine in figure 4. Smaller and smaller quantities of Rhodamine were added to a suspension of colloid. Eventually, a concentration of Rhodamine in colloid which would effectively be 6 x 10-18 M if no Rhodamine was adsorbed on the surface was obtained. This corresponds to approximately 20-40 molecules present in the laser beam at any one time. The experiment was not limited by the sensitivity of the instrument, but was instead limited by the difficulty of dealing with contamination problems at this level. It will be noted that the concentration dependence is not linear and this may well be because of a desorption/adsorption process occurring between the glass and silver surfaces and the solution. At this point in time, the main purpose of this diagram is to illustrate the enormous sensitivity which is possible. Above monolayer coverage, the spectra are affected by fluorescence from non-attached dye molecules.
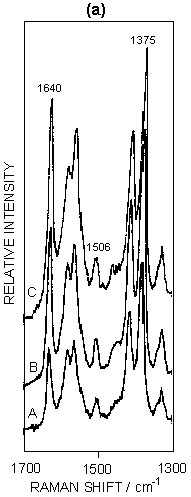
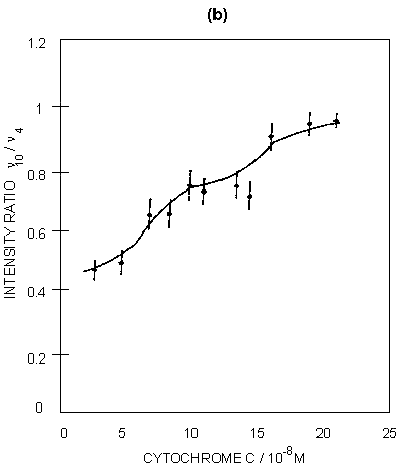
Figure 5: (a) SERRS spectra of ferricytochrome c at concentrations of: (A) 4 x 10-8M; (B) 7.5 x 10-8M; (C) 1.5 x 10-7M. Spectra were recorded 30 minutes after aggregation using 514.5 nm excitation. (b) Graphical illustration of the intensity ratio of v10 to v4 with increasing surface coverage.
SERRS has also been used to study biological molecules; for example, studies of enzymes on proteins are well known. An illustration is given in figure 5 for cytochrome c adsorbed on silver colloid. The intense bands in the spectra correspond to those found in resonance, but the relative intensities do vary when compared to resonance. The band at 1375 cm-1 (v4) is a marker for the oxidation state and v3 at 1506 cm-1 is a marker for the spin state. By gradually titrating cytochrome c into a silver colloid, adsorption layers on the surface have been built up from below to above monolayer coverage. When this occurs, there is a relative intensity change in a number of the bands. Specifically, the ratio of the intensity of the band at 1375 cm-1 (A1g, v4) to the band at 1640 cm-1 (B1g, v10) changes. By plotting this intensity the formation of the monolayer can be observed. The reason for this change is that SER(R)S, provided the effect contains an appreciable SERS contribution, is dependent on selection rules related to those obtained from SERS alone. Thus, as the monolayer builds up on the surface, the protein packs and the orientation of the haem in the protein relative to the surface changes. This change is reflected in the relative intensity changes between v4 and v10. In fact at close to monolayer coverage more subtle reorganisation of the layer occurs, as shown in figure 5.
81. Analytically, the first papers on quantitative SERRS are beginning to appear. They require very careful control of the aggregation and the use of adsorbates which ensure that there is efficient dye-metal surface adhesion [10, 11]. Additionally, it must be recognised that the field gradients in aggregated colloid vary within the aggregate and consequently even distribution of the adsorbate is essential.
Furthermore, for quantitative use it is essential that concentration dependence calibration curves and relative standard deviation (RSD) values are reported. Many reported SERRS experiments do not give these so it is difficult to evaluate the results. To aid the reliability of quantitative SERRS a new series of dyes designed to complex onto the silver surface have been produced. With these dyes RSD values of between 2 and 4 % have been obtained using a Raman microscope. Given that RSD values of 2 % are normally obtained for Raman spectra with this instrument; this suggests that quantitative SERRS is possible.
82. An alternative approach of value for qualitative analysis is to coat colloid onto a suitable coloured surface. This procedure has been used to determine in situ, in a single cotton fibre, the nature of the covalently bound dye [12]. The intention of the work was to provide a rapid test for use in forensic science to match a fibre found at a crime scene to a garment.
This review has concentrated on SERRS applications using colloid, but many other applications are currently being developed; for example, it is possible to use evaporated silver to determine the nature of thin films of non-linear optic materials [13]. Further, electrodes are used to provide SERRS detection for chromatography [14]. The advantage of the electrode system is that the electrode surface can be regenerated between determinations.
83. Conclusion
SERRS has been shown to be an extremely specific and sensitive technique with additional advantages over the previous techniques of SERS and resonance Raman. It has been shown to approach fluorescence in sensitivity and applies to a wider range of materials than resonance Raman. However the technique does have the limitations that a suitable chromophore must be present in the molecules to be studied, and the molecule must be in proximity to a roughened surface of an appropriate metal.
84. References
1. T.M. Cotton, J.H. Kim and G.D. Chumanov, J. Raman Spectrosc., (1991), 22, 729
2. C.H. Munro, W.E. Smith, D.R. Armstrong and P.C. White, J. Phys. Chem., (1995), 99, 879
3. K. Xi, S.K. Sharma, G.T. Taylor and D.W. Muenow, Appl. Spectrosc., (1992), 46, 819
4. M. Moskovits, Rev. Mod. Phys., (1985), 57, 783
5. R.J.H. Clark and B. Stewart, Struct. Bonding (Berlin), (1979), 36, 1
6. P.C. Lee and D. Meisel, J. Phys. Chem., (1982), 86, 3391
7. C.H. Munro, W.E. Smith, M. Garner, J. Clarkson and P.C. White, Langmuir, (1995), 11, 3712
8. C. Rodger, W.E. Smith, G. Dent and M. Edmondson, J. Chem. Soc. Dalton Trans., (1996), 791
9. K. Kneipp, Y. Wang, R.R. Dasari and M.S. Feld, Appl. Spectrosc., (1995) 49, 780
10. J.J. Laserna, Anal. Chim. Acta, (1993), 283, 607
11. C.H. Munro, W.E. Smith and P.C. White, Analyst, (1995), 120, 993
12. P.C. White, C.H. Munro and W.E. Smith, Analyst, (1996) 121, 835
13. J. McAleese, B.N. Rospendowski, D.B. Sheen, J.N. Sherwood and W.E. Smith, J. Raman Spectrosc., (1997) 28, 73
14. V.J.P. Gouveia, I.G. Gutz, J.C. Rubim, J. Electroanaly. Chem., (1994), 371, 37
REF: Int. J. Vib. Spect., [www.irdg.org/ijvs] 1, 4, 68-84 (1997)
