Dear Readers (Volume 5 Edition 2)

Geoff Dent has been in contact and asked us why we have no searchable index of IJVS. The answer is laziness – I just have not had the time to create it. Leave it with me folks I will mend my ways!! [Great something else for me to nag you about! – Louise]
We had an email from Michael Barnett from Princeton in the States.
I would be interested in an answer to the following question. Are there problems of current interest in the theory of vibrational spectra which could be tackled by a technique that proceeds from a secular equation containing symbolic parameters ( as well as numbers) in the matrix elements, to a solution that expresses the roots of the equation as power series in these parameters (or in further variables that these parameters depend on)? I have worked through the practicalities of doing this for a formally similar problem involving matrices of order 125, for a different chemical topic, and I am interested in further applications.
To be honest Michael, I have no idea, but I’m sure some of the readers will have an opinion. So folks – please comment with copies to the Editorial Office. Michael’s email is michaelb [at] princeton.edu. Perhaps our contributor Prof. Bové [see section 2] will have a view.
Next in our last edition Tom Perrone had a query about potential reviews in a number of areas, including sampling accessories for FT-IR, FT-Raman and FT-NIR. Well we’ve had a response from Andrew Hind from Australia who says.
I have recently co-authored an article reviewing the theory of FTIR/ATR spectroscopy and its use in interfacial spectroscopy which will soon be published in Advances in Colloid and Interface Science. If Tom (or anybody else for that matter) is interested in obtaining an advance copy (of the proof) they can contact me at andrew.hind [at] varianinc.com
Thanks Andrew, also Tom has responded to another query, one from Noel Franck, in the last edition. Noel was asking about the availability of a spectrum database of gases in NIR.
Noel, please find a preliminary answer to your inquiry in the recent paper by Chris Brown and Jing Zhou found in a recent edition of Applied Spectroscopy. Appl. Spec., 55, No 1, 44 (2001). These authors may be able to provide you with more information.
An email from David Edwards recently asked the following.
I have a Perkin Elmer Spectrum 2000 with an I-Series microscope. The instrument has a facility to polarise the IR beam. There is a dearth of explanation as to its use. I am trying to measure the degree of draw/orientation of nylon fibres using a diamond compression cell. The one major problem is how do I know with certainty the angle of orientation of the polarised light impinging the specimen and will the diamond windows affect that orientation.
The classical method of checking the polarisation is to use 2 polarisers – not a cheap idea but in the case of a conventional ATR system you can use a polariser and a piece of oriented plastic.
I would use a piece of drawn polypropylene. When the film is rotated a minimum in one or two bands can be found hence checking the rotational effect of the ATR accessory. I would be very doubtful that any rotation occurs in a conventional ATR.
Turning to the microscope – far more potential for errors, I would think. How about making a very thin highly oriented polymer specimen and in effect putting it over a mirror? i.e. recording the spectrum by double transmission?
The set-up would be –
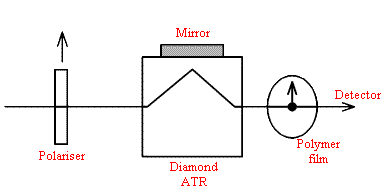
I would use as gentle pressure as possible, rotate the film about the optical axis and look for minima in band intensities.
Polymer sample – supermarkets usually supply meat or cheese in very thin plastic bags that rustle when squashed and have a very low coefficient of friction. They are made of linear (Hi Density) polyethylene. Carefully cut a strip ~1cm wide. Make sure there are no nicks in the edges. Grip the film between your thumbs and first fingers and gently stretch the film. You can, if you are lucky extend the length x 10. The film will be highly oriented and VERY thin. Good Luck.
Now quite a long question from Dr Simona Badilescu from Canada. Simona writes..
Would you believe that I read your last Editorial while struggling to make sense of my infrared data in polarized light? As you are complaining about the scarcity of IR work in polarized light, and as I am preparing an article precisely for the IJVS, would you be kind to think about my problem? But you would probably like to know something about me and my background. When I will tell you that my first instrument was not a Hilger H800 but an UR10 Zeiss prism instrument (back in Romania), you will understand that I am not a new practitioner. After the UR10 – a very robust two-beam instrument, with three prisms and a plotter with needles scratching on a waxy paper, (yes, maybe the spectra were noisy with weak bands but at least they were true, much more than today’s ratioed, smoothed, base-line corrected, fitted perfect FTIR spectra), I had the chance to work with some very good Perkin-Elmer grating instruments (from Uberlingen in Germany) and only in the late 80s, in Canada, I had my first FTIR. Now, for a number of years, I do what is called “thin film spectroscopy,” i.e. the preparation and spectral study (mostly by ATR) of transition metal oxide films for electrochromic and photonic applications. But this is history, and you are certainly more interested in my recent work that prompted this letter.
We are using polystyrene spheres (monodisperse samples with diameters in the nanometer range and with charged functional groups on the surface) to prepare various photonic materials. Under certain conditions these spheres may crystallize into an ordered array (fcc), resulting in an iridescent sample with optical properties similar to high quality opals. The aim of my work is to correlate the degree of ordering (from AFM and SEM studies) with the orientation as found from IR data in polarized light. I used materials such as Si wafers, ZnSe ATR crystals (this was used without polarizer) but also some polymer films as substrates for these colloidal crystals. The dichroic ratio of the “aromatic” bands (1600 and 1493 cm-1) but also of the 710 cm-1 CH2 rocking, in samples ordered on Si and polystyrene films (measured by rotating the polarizer, not the sample) show, indeed, a nonrandom orientation of the molecules on these substrates. In these cases, the SEM images show a very good ordering. When the sample is not ordered, IR data point toward a random orientation (D=1). I have some difficulty to understand how linear polystyrene macromolecules can be oriented in spherical particles sticking to the substrate. One explanation would be a slight deformation of the spheres due to the strong intersphere compression (this is visible in some images). What do you think? Is there something else what I should do to ascertain the results? I am now depositing the colloidal crystals on a Si wafer covered by a thin SiO2 layer (deposited by a sol-gel method) to see the effect on D. I would be grateful for any ideas you may have on this work.
I cannot end this message without telling you how much I enjoy the Journal. Reading it is like coming home, among people who speak a language you can easily understand. It is a pleasure, and I would like to thank you and Louise for building this place and letting us come in.
Thank you very much Simona for your very nice remarks about IJVS, it is always good to hear that we are appreciated. I may be a bit stupid but do I understand that your spheres are packed in a face centred cubic array and are coated onto crystalline substrates (Si or ZnSe). Since the spheres are spherical and the packing FCC there cannot be any preferred orientation. However, we know that adsorption to crystalline substrates can cause epitaxial crystallisation but the epitaxial layer is usually very think (200 Å or so).
You mention that these materials are opalescent. Why? Could this be the origin of your dichroic behaviour? Do these materials have defects in layers perhaps leading to orientation? You don’t say what D values you get. Can you give me some idea of typical values.
We will publish this correspondence in the Journal and ask readers if they have any ideas but it might help to give them a little more information. Simona’s email if any of you have any thoughts is badiles [at] umoncton.ca
Finally a question from D.C. Young from the States..
Thanks for the guide to the intensity of spectral bands. What is the “standard” cell path used for this classification?
The point about my piece is the subjective nature of the intensity descriptions. The path length (and/or concentration) should always be adjusted so that the strongest bands are VERY strong. In this way the VERY weak bands are clearly visible. Now in infrared absorption spectroscopy, the absolute absorption ( the extinction coefficients) can vary over a large range so there can never be a standard pathlength.
That’s it for this edition. Keep your questions coming in and if you answer any of the questions raised , don’t forget to copy us at the Editorial Office.
