SERS Studies of Polymer Surfaces and Polymer/Metal Interfaces
Gi Xue* and Yun Lu
Department of Polymer Science & Engineering,
Nanjing University,
Nanjing, 210093,
P.R. China
*Email: p709 [at] netra.nju.edu.cn
Abstract
Surface Enhanced Raman scattering for the polymer/metal interface and for the polymer film surface were performed on nitric acid-etched silver or copper and by chemically deposited colloidal silver, respectively. SERS measurements for samples of current interest, such as polymer/metal interface, nanometer diameter fibre, and liquid crystal film were successfully performed without disturbing the surface morphology. The results of this work show that our SERS sampling techniques are particularly effective.
Keywords
Surface enhanced Raman scattering, nitric acid-etched foil, chemically deposited silver, surface, film, fibre
Introduction
Polymer films cast from solution onto a metal surface usually form multilayers of the macromolecular chains. When the cast solution is diluted to produce low coverage and hence thin films, the adsorbed polymer is prone to form islands or clumps upon evaporation of the solvent, resulting in an inhomogeneous surface under analysis. So, the film of polymer on metal must be a few hundred angstroms thick in order to cover the surface entirely. The interfacial region is restricted to a few or a few tens of angstroms of polymer adjacent to the metal surface [1]. Hence it represents only a small proportion of the bulk layer and it is therefore difficult to measure the interfacial region without the interference of the polymer bulk. Until recently, process technology had developed largely empirically without any specific knowledge of the nature of chemical bonding at the interface. With the advent of more sophisticated surface analytical techniques, polymer/substrate interaction has begun to be studied on a more detailed molecular level. Among the various state-of-the-art tools in surface analysis, X-ray photoelectron spectroscopy (XPS) is used to investigate core level shifts of the polymer components, as well as those of the metal atoms themselves in order to elucidate the nature of the bonding between metal and polymer. However, the uncertainties inherent in XPS analysis of polymer/metal interactions stem mostly from differential charging of the sample surface during an XPS measurement [2]. Differential charging leads to artifacts in the XPS spectra, which may be mistaken for chemical bonding effects.
Metals can be studied by the use of electron spectroscopy, but only if the polymer layer is absent or is less than 3-5 nm thick because of the limitation of the mean free paths of the photoemitted electrons in the energy range of interest. However, it is difficult in practice to cast synthetic polymers as a uniform monolayer film i.e. with thicknesses less than a few tens of angstroms onto metals. In the manufacture of coatings or adhesives on metals, a ‘thin’ layer may mean a thickness of hundreds of nanometers. Infrared (IR) reflection-absorption and diffuse reflectance spectroscopy provides rich information about the surface film [3-6], but almost exclusively from the polymer bulk. Both IR and normal Raman spectroscopy could hardly observe the structure of a zone 10-30 Å thick of polymer near the surface without the interference of the bulk polymer that is at least hundreds of angstroms thick. As a result of the difficulty of the experimental measurement on the microstructure at the interface, our specific knowledge of the physical chemistry, such as surface bonding, molecular orientation, catalyzation under various conditions, diffusion of metal ions, and the electrochemical properties in the interfacial region, is only poorly understood. It is therefore necessary to develop new techniques for as to investigate the interfacial structure minimizing the interference of the bulk polymer.
The discovery of surface-enhanced Raman scattering (SERS) is probably one of the most important developments in the area of surface science in the last two decades [7-9]. Enhanced Raman spectra that are 105-106 times as strong as normal Raman scattering were reported [10]. Boerio pointed out that SERS is good for the study of polymer-silver island interfaces since it enhances almost exclusively the first monolayer of molecules adjacent to the metal. Thus the technique makes it possible to examine the interfacial region between metal and polymer as long as the polymer films are not so thick that normal Raman scattering from the bulk of the film is more intense than SERS from the interface [11].
Since the original SERS work involving pyridine adsorbed on silver electrodes and sols, most of the reported SERS spectra of polymers were recorded from electrochemical cells, aqueous environments, or in vacuum [12-15]. It has been reported that spin-casting of a polymer solution onto Ag island films could be characterized by SERS [11,16,17]. However, we found that the SERS effect of vacuum-deposited Ag island film is not stable at elevated temperatures and that copper substrates prepared by this method show little SERS effect on the adsorbed species. This disadvantage has limited the application of SERS as a general tool in the analysis of the polymer-metal interface, since many composites must be processed or be in used at elevated temperatures. Recently, HNO3-etched silver and copper foils for Raman study of adsorbates were prepared in this laboratory and in others [18,19]. This new sampling technique exhibits a strong SERS effect on surface adsorbates and improved thermal stability [20]. Thin-layer coatings made by solution casting can be investigated on the newly-developed Raman enhancing Ag and Cu substrates, in order to elucidate the adsorption and desorption of polymer chain and catalyzation, at the interface and to study the oxidation of metal under the polymer overlayer.
Enhanced Raman scattering spectra can be now easily collected from the samples adsorbed on SERS active substrates. However, few experiments have been made on the surface studied for a fibre or a film by the SERS technique. Recently, we have developed an effective SERS technique for characterizing the surface structure of film or fibre, without disturbing the morphology of the samples. The preparation of SERS-active over-casting layers involves the use of a silver colloidal deposition method via the reduction reaction of AgNO3 in deionized water at room temperature. The silver colloids are directly deposited onto the surface of a film or fibre to form an SERS-active over-coating layer. The incident laser radiation penetrates through the silver over-coating layer and reaches the surface of the samples and the scattered radiation that contains the information on the film surface was collected and then analyzed. On the other hand, when the colloidal silver is chemically deposited onto glass, a SERS-active substrate can be prepared for the study of adsorbed materials. To our surprise, such substrates show extraordinary stability under a wide range of environmental conditions. Good thermal stability of the surface enhancement factor is very important in order to apply SERS to catalytic studies, since many chemical reactions are undertaken at elevated temperatures.
The Study of Polymer/Metal Interfaces
Preparation of SERS-Active Metal Substrates
Commercial copper foils of 0.025 mm thickness were immersed into 2 M nitric acid solution at 30ºC. After about 10 s, a number of bubbles were formed near the surface of copper. Vigorous agitation was then applied. After 2 min, a sponge-type surface with substantial roughness was created. In the case of etching silver foils, 5-6 M HNO3 was used and the etching time was prolonged to 5-10 min until the surface became milky. The roughness of the etched metal surface was in the range 10-100 nm as measured by electron microscopy [18]. After etching, the metal foils were thoroughly rinsed with water and dried in air. The etched metal foils were then ready for polymer sampling.
Raman spectra were recorded with a SPEX-1403 Raman spectrometer. The incident laser excitation was 647.1 nm from a Kr+ laser source with an output of 40-100 mW. A back-scattering geometry in air was used for all samples. The foils were mounted onto a heating cell for the in situ Raman measurement at elevated temperatures.
Thermal Stability of SERS-Active Substrates Covered with Polymer Film
Figures 1 and 2 compare the thermal stability of the surface enhancement factors of the nitric acid etched silver and copper foils and the vacuum deposited silver island film. The Raman band intensities are reported in counts S-1mW-1 to account for the different laser power and integration times. In order to ensure that there is no laser-induced change in the SERS spectra, the foil was spun during the spectrum measurement. Spinning also averages the detecting adsorbate thickness. By comparison of the Raman line intensities and the signal-to-noise ratio of spectra at Figures 1 and 2, it is clear that the HNO3-etched silver or copper foils will produce good SERS spectra for polybenzimidazole at elevated temperatures, while the vacuum-deposited silver island films lose most of their enhancement. The order of Raman enhancement factor at elevated temperature can be arranged as HNO3-etched Ag > HNO3-etched Cu > Ag/CaF2. The good enhancement factor and high stability of the HNO3-etched metal foils indicate that SERS has real potential for use as an in situ analytical tool for the study of interfacial structure at elevated temperature.
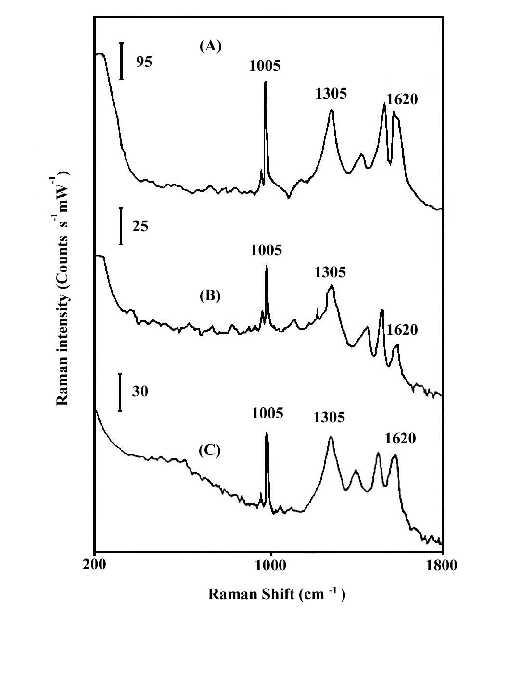
Figure 1. In-Situ SERS spectra of polybenzimidazole at 100°C.
(A) on HNO3-etched silver foil;
(B) on vacuum-deposited Ag/CaF2,
(C) on HNO3-etched copper foil.
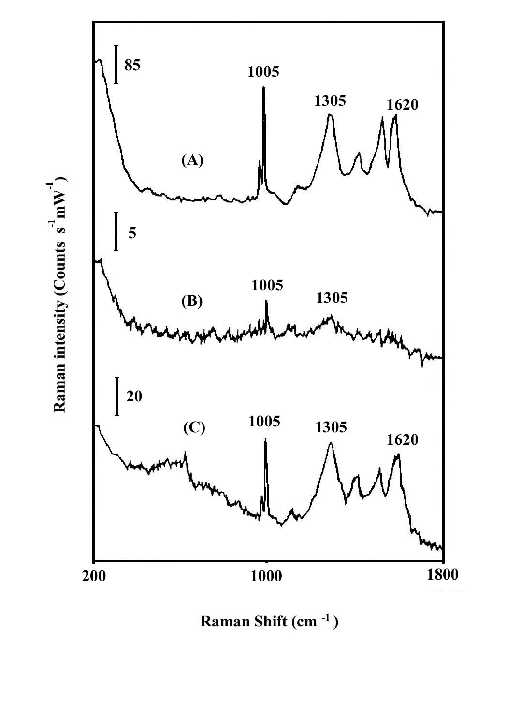
Figure 2. In-situ SERS spectra of polybenzimidazole at 200°C.
(A) on HNO3-etched silver foil;
(B) on vacuum-deposited Ag/CaF2;
(C) on HNO3-etched copper foil.
Observation of Adsorption and Surface-catalyzed Reaction of Polyacrylonitrile on Silver.
The interaction of the cyanide side group of polyacrylonitrile (PAN) with metal surfaces is important from both theoretical and practical (catalytic) points of view. However, it is only recently that detailed studies of CN surface chemistry have appeared [21-27]. In the IR spectrum, the C=N stretching vibration of a chemisorbed sample on Cu/SiO2 was appreciably shifted to lower frequency regions in comparison with that in liquid state, suggestive of significant coordination of the cyano group in acrylonitrile with the Cu surface site [28]. Our SERS studies of the polymer on silver foil provide information concerning adsorption-induced cyclization at room temperature. Figure 3A shows a normal Raman spectrum of polyacrylonitrile spin cast coatings of about 3000 nm thickness on a smooth silver foil. Figure 3B and C show SERS spectra recorded from polyacrylonitrile coatings on HNO3 etched surfaces where the samples were prepared by spreading 20 µl of a 0.3% solution on 2 cm2 foils followed by fast and slow evaporation of the solvent, respectively. The band at 2245 cm-1 of Figure 3B, having the same position and band width as that in Figure 3A, can be assigned to freely dangling CN groups of polyacrylonitrlie. A broad band centered at 2160 cm-1 of Figure 3B is also attributed to the C=N stretching mode. The substantial shift (85 cm-1) and band broadening upon adsorption indicate the direct interaction (side-on coordination) between the cyano groups and the silver surface atoms. Here, the presence of side-on coordination of CN groups of polyacrylonitrile on Ag corroborates Kim’s proposal that this type of interaction is favored when the CN group is not conjugated with an unsaturated group [29]. A similar phenomenon has been observed in the case of aliphatic dinitriles adsorbed on Cu surfaces in other SERS studies [30]. Therefore, the 2160 cm-1 band implies that the coordination of Ag surface atoms to the cyano groups brings about back-conation of electrons from the metal to the CN antibonding orbitals, therefore reducing the CN triple-bond order. This type of adsorption on the roughened surface weakens the CN triple bond and facilitated the adsorption-catalyzed cyclization under mild conditions. The SERS spectrum of Figure 3C is typical of aromatic ring vibrations and correlates well with the FTIR spectra of degraded PAN [31]. The bands at 1600 and 1580 cm-1 can be assigned to –(C=C)- or –(C=N)- conjugation ring stretching vibration, implying that the adsorbed PAN has been turned into a fused ring structure at the interfacial region. This is consistent with the signal intensity of the 2245 cm-1 band. The bands at 1080 and 1000 cm-1 can be ascribed to in-plane ring C-H deformation frequencies. Their strong intensity, leads to the reasonable proposal that the aromatic ring structure is sticking up on the metal surface via its N atoms or at least the fused rings are tilted off the surface a proposal based on the electromagnetic theory of the SERS selection rule [32]. A postulated schematic diagram of the adsorption and catalyzed cyclization of polyacrylonitrile at the polymer-metal interfacial region is shown in Fig.4.
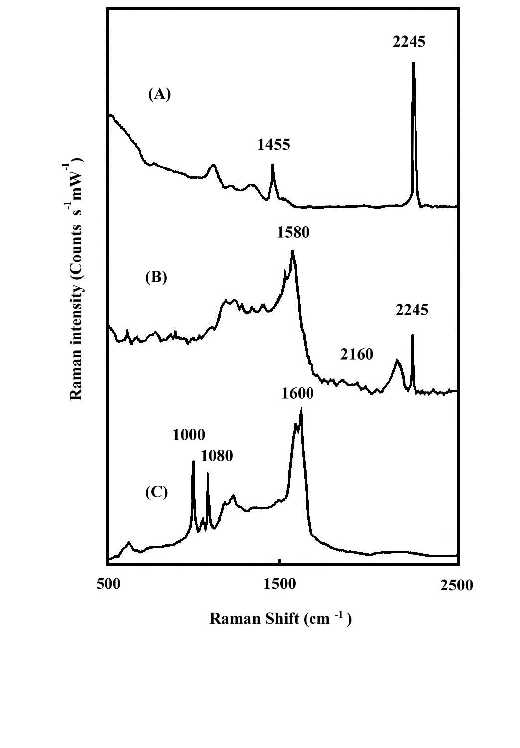
Figure 3. (A) Normal Raman spectrum of polyacrylonitrile (PAN) on smooth silver foil;
(B) SERS spectrum of PAN on rough silver foil. The film was prepared by fast evaporation of the solvent;
(C) SERS spectrum of PAN on rough silver foil. The film was prepared by slow evaporation of the solvent.
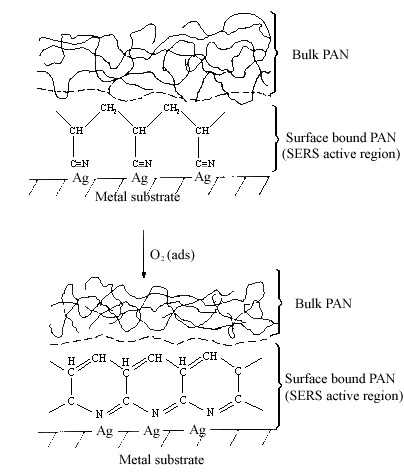
Figure 4. Schematic diagram of polyacrylonitrile adsorption and cyclization.
Chemically Deposited Silver Used as a SERS-active Over-coating Layer for Polymer Films and Fibres
The silver over-coating layer was prepared by depositing silver particles by the “Tollen’s test” method onto the polymer film under investigation. A piece of test film 10×10 nm in size was placed in a 10ml beaker to which 5ml 0.05 M silver ammonia complex and 2ml formaldehyde were added and mixed. A few seconds after mixing, the solution turned to grey and then black. The silver ions reduced to form colloidal particles and are deposited onto the test film to form an over-coating layer at room temperature. After withdrawing the test film covered with its silver over-coating, it was washed with distilled water and dried. Then the specimen was ready for Raman studies.
The study of over-coated polymer film
Two pieces of polystyrene (PS) film 5 micron thick were prepared by evaporating 3 wt% and 0.2 wt% solutions in benzene, respectively. Silver over-coating layers were deposited chemically onto these films. Laser radiation penetrates through the silver over-coating layer and interacts with the silver/polymer interface, the scattered radiation as collected from the silver over-coating layer (Figure 5). Figure 6 shows the SERS spectrum (6A) collected from the silver over-coating layer on the polystyrene film prepared from 3 wt% solution, the spectrum (6B) for the neat chemically deposited silver substrate, and the difference spectrum (6C), respectively. One can easily identify the vibrations associated with phenyl rings including the bands at 3055, 1603, 1032, and 1037 cm-1, and the bands associated with backbone C-H stretching at 2902 and 2851 cm-1. Figure 7 presents the corresponding spectra collected from the polystyrene film prepared from 0.2 wt% solution. Although the thickness of the film was the same as the sample in Figure 6, the SERS spectra are quite different. In Figure 7, the C-H stretching band at 3055 cm-1 and the ring stretching are at 1603 cm-1 are significantly reduced in intensity. However, the ring out-of-plane vibration at 760 cm-1 was enhanced. These spectral differences indicate that the molecular orientation with respect to the surface of the film prepared from the dilute polystyrene solution is different from that from the more concentrated solution, and for the former, the phenyl rings tend to “lie down” flat on the surface (Fig.8). Thus it seems that the chemically deposited over-coating silver can be used as a sensitive SERS technique for surface analysis of a film.
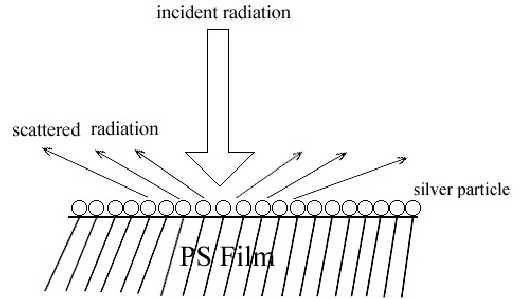
Figure 5. Schematic diagram of the incident radiation and scattered radiation on polystyrene film coated with silver mirror
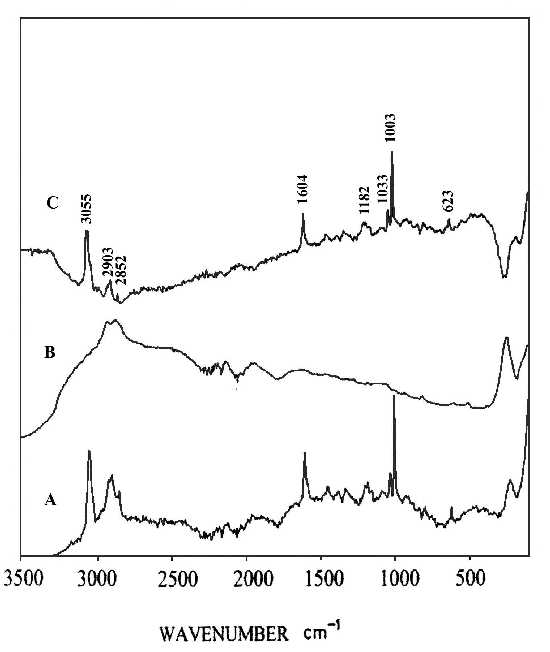
Figure 6. (A) SERS spectrum of silver over-coating layer on polystyrene film prepared from 3 wt% solution,
(B) SERS spectrum of a chemically deposited silver film,
(C) difference spectrum of A and B.
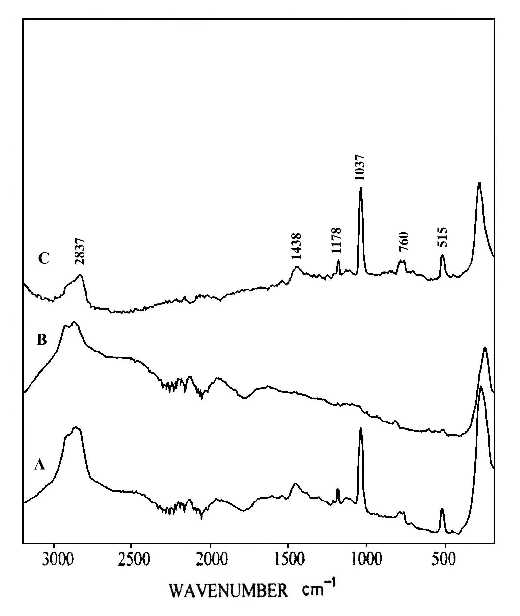
Figure 7. (A) SERS spectrum of silver over-coating layer on polystyrene film prepared from 0.2 wt% solution,
(B) SERS spectrum of a chemically deposited silver film,
(C) difference spectrum of A and B.
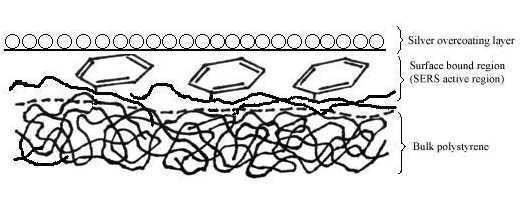
Figure 8. Schematic diagram of the surface structure of polystyrene film prepared from 0.2 wt% solution under the silver over-coating layer.
The study of over-coated polymer fibres
Polyacrilonitrile fabrics made of nanometer diameter fibres produced by electro-spinning were chemically deposited with silver film. The SERS spectrum is shown in Figure 9B. Figure 9A is a normal Raman spectrum of a polyacrilonitrile film. Figure 9B contains a characteristic band at 2245 cm-1, which is similar to the pure polyacrilonitrile. The new bands in the SERS spectrum (Figure 9B) are probably due to pyrolysis of the fibre under the laser radiation [33,34].
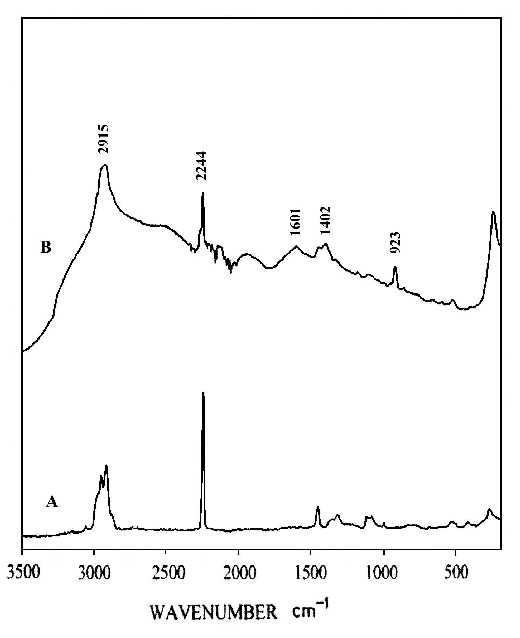
Figure 9. (A) Normal Raman spectrum of polyacrilonitrile film,
(B) SERS spectrum of silver over-coating layer on polyacrilonitrile fabrics of nanometer diameter fibres by electrospinning.
SERS studies on the rubbing effect in a liquid crystal film
Liquid crystal alignment, which is essential for liquid crystal display technology, is often realized by using rubbed polyimide films as an aligned layer [35]. Therefore, it is important that we understand the mechanism responsible for this surface-induced liquid crystalline alignment, including the possibility of controlling the pretilt angle. The pretilt angle means the initial orientation (state) of the liquid crystal molecule. When an external signal (voltage) is applied to the liquid crystal, this initial angle of orientation will change to function the liquid crystal display[36,37]. This suggests the importance of knowing the molecular orientation and chemical structure of the polyimide surfaces. Most of the studies in this area rely on bulk-sensitive methods such as infrared dichroism and optical retardation. Recently, some surface-sensitive measurements using electron spectroscopy for chemical analysis (ESCA) and X-ray scattering to obtain molecular orientation and structure of this type of polyimide surfaces have been reported [38-40]. A clear understanding of the correlation between polyimide surface structure and bulk liquid crystal alignment is still missing, suggesting the need for further investigation with other surface sensitive experimental techniques. In this study, we report a SERS invenstigation of a polyimide, which has a cyano group in the side chain, especially designed to generate high pretilt liquid crystal alignment. Thin films of the polyimide for our measurements were made by spin-coating a 2 wt% solution in cyclopentane on to a glass substrate followed by baking at 120ºC for 20 h. The thickness of the film was controlled so that it lay in the range 3-5 micron. Rubbing was carried out with a rotating drum wrapped with velvet. Silver colloids were directly deposited on to the rubbed and also the un-rubbed surfaces of the polyimide film simultaneously. Figure 10A shows a normal Raman spectrum of the polyimide film, which shows characteristic bands for the cyano group at 2227 cm-1 and for the aromatic rings at 1606 cm-1. Figure 10B and C illustrate the SERS spectra for an un-rubbed polyimide liquid crystal film and for the rubbed film, respectively. The SERS spectrum (Figure 10B) for the non-rubbed film is quite different from the bulk film in that the intensities of the 2229 and 1606 cm-1 bands and for those near 920 and 610 cm-1, indicate that molecular alignments at the polyimide surface are different from those in the bulk. Figure 10C show spectra from the rubbed film. Although the relative intensities are different because of the selection rules associated with surface enhancement on metals, the 2229 and 1606 cm-1 bands are clearly present in the SERS spectrum. Here we can clearly see that the surface structures of the polyimide film are different before and after rubbing, indicating that the rubbing process significantly rearranges the surface orientation distribution of the side-chains, and slightly changes the alignment of the backbone. This result may shed some light on our understanding of the molecular mechanism responsible for aligning liquid crystal by rubbed polyimide and the systematic design of alignment layers for inducing high-pretilt liquid crystal bulk alignment.
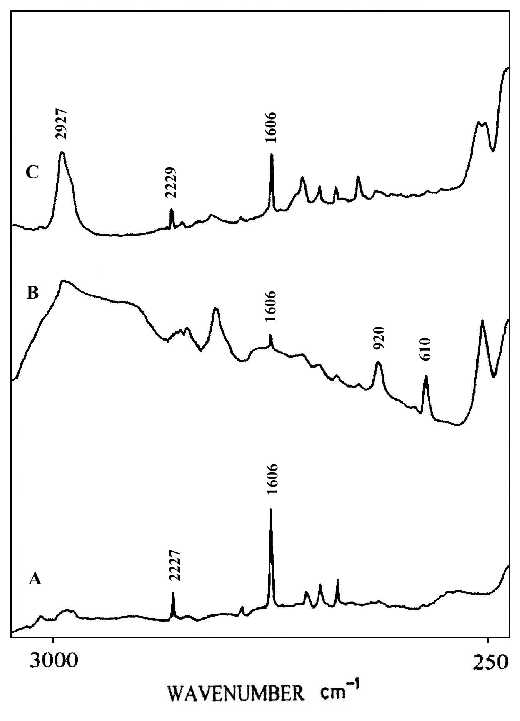
Figure 10. (A) Normal Raman spectrum of a liquid crystal polyimide film,
(B) SERS spectrum for a silver over-coating layer on the non-rubbed polyimide liquid crystal film,
(C) SERS spectrum for the silver over-coating layer on rubbed film.
References
- Ishida, H., Ed. Controlled Interphase in Composite Materials; Elsevier: New York, 1990.
- Pertsin, A.J; Pashunin, Yu. M. Appl. Surf. Sci. 44, 171 (1990).
- Koenig, J. L. Spectroscopy of Polymers; American Chemical Society: Washington, DC, 1992.
- Jolley, J. G.; Geesey, G. G.; Hankins, M. R.; Wright, R. B.; Wichlacz, P. L. Appl. Spectrosc. 43, 1062 (1989).
- Dowrey, A.E.; Marcott, C. Appl. Spectrosc. 36(4), 414(1982).
- Xue, G.; Liu, S.; Jin, Y.; Jiang, S. Appl. Spectrosc., 41(7), 1172 (1987)
- Fleischman, M.; Hendra, P. J.; McQuillan, A. J. Chem. Phys. Lett. 26,163 (1974).
- Jeanmaire, D. J.; Van Duyne, R. P. J. Electroanal. Chem. 84,1 (1977).
- Albrecht, M.; G.; Creighton, J. A. J. Am. Chem, Sco. 99, 5215 (1977).
- Chang, R., F., Eds. Surface Enhanced Raman Scattering; Plenum Press: New York, 1982.
- Boerio, F. J. Thin Solid Films, 181, 423 (1989).
- Heart, S. M.; Grieser, F.; Barraclough, C. G. Chem. Phys. Lett. 95, 154 (1983).
- Lee, P. C.; Meisel, D. Chem. Phys. Lett. 99, 262 (1983).
- Barnickel, P.; Wokaun, A. Mol. Phys. 67, 1355 (1989).
- Tashiro, K.; Matsushima, K.; Kobayashi, M. J. Phys. Chem. 94, 3197 (1990).
- Boerio, F. J.; Tsai, W. H.; Montaudo, G. J. Polym. Sci., Part B: Polym. Phys.27, 1017 (1989).
- Venkatachalam, R. S.; Boerio, F. J.; Roth, P. S.; Tsai W. H. J. Polym. Sci., Part B:Polym. Phys. 26, 2447 (1988).
- Xue, G.; Ding, J.; Zhang, M. Chin. Sci. Bull. 36, 194 (1991).
- Carron, K. T.; Xue G.; Lewis, M. L. Langmuir 7, 2 (1991).
- Xue G.; Dong, J. Anal. Chem. 63(20), 2393 (1991).
- Kordesch, M. E.; Stenzel, W.; Conard, H. Surf. Sci. 186, 601 (1987).
- Sexton, B. A.; Avery, N. R. Surf. Sci. 129,21 (1983).
- Kishi, K.; Okino, Y.; Fujimoto, Y. Surf. Sci. 176, 23 (1986).
- Shanahan, K. T.;Muetterties. E. L. J. Phys. Chem. 88,1996 (1984).
- Hemminger, J.;Muetterties, E. L.;Somorjar, G. A. J. Am. Chem. Soc. 101, 62 (1979).
- Friend, C. M.; Muetterties, E. L.; Gland, J. L. J. Phys. Chem. 85, 3256 (1981).
- Solomum, T.; Christmann, K.; Baumgartel, H. J. Phys. Chem. 93, 7199 (1989).
- Sigiyama, K.; Miura, H.; Sekiwa, H.; Matsuda, T. Chem. Lett. 2093 (1986).
- Chun, H. A.; Kim, M. S.; Kim, K. J. Mol. Struc. 221, 127 (1990).
- Loo, B. H.; Lee, Y. G.; Frazier, D. O. Chem. Phys. Lett. 119, 312 (1985).
- Colman, M. M.; Petcavich, R. J. J. Polym. Sci., Polym. Phys. Ed. 16, 821 (1978)
- Creighton, J.A. Surf. Sci. 124, 209 (1983).
- Xue, G.; Dong, J.; Zhang, J. Macromolecules, 25, 5855 (1992).
- Xue, G. Prog. Polym. Sci., 22, 313 (1997).
- Scheffer, T. J.; Nehring, J. J. Appl. Phys. Lett., 45, 1021 (1984).
- Feller, M. B.; Chen, W.; Shen, Y. R. Phys. Rev., 43, 6778 (1991).
- Zhuang, X.; Marrucci, L.; Shen, Y. R., Phys. Rev. Lett., 73, 1513 (1994).
- Seo, D.-S.; Kobayashi, S.; Nishikawa, M. Appl. Phys. Lett. 61, 2392 (1992).
- Toney, M. F.; Eussel, T.P.; Logan, J. A. Kikuchi, H.; Sands, J. M.; Kumar, S. K. Nature, 374, 709 (1995).
- Samant, M. G.; Stohr, J.; Brown, Russell, T. P.; Sands, J. M.; Kumar S. K. Macromolecules, 29, 8334 (1996).
REF: G. Xue and Y. Lu, Int. J. Vib. Spect., [www.irdg.org/ijvs] 4, 2, 5 (2000)