Reflection Spectra
17. Reflection Spectra
Dr Richard Spragg
Most materials absorb infrared radiation very strongly. As a result samples have to be prepared as thin films or diluted in non-absorbing matrices in order to measure their spectra in transmission. There is no such limitation on measuring spectra by reflection, so that this might look like a more versatile way to obtain spectroscopic information. However reflection spectra often look quite different from transmission spectra of the same material. Here we shall look at the nature of reflection spectra and see when they are likely to provide useful information.
18. The nature of reflection spectra
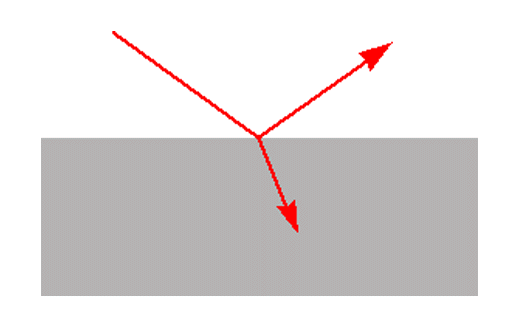
Figure 1. Reflection and transmission at a plane surface
Reflection takes place at surfaces. When radiation strikes a surface it may be reflected, transmitted or absorbed. The relative amounts of reflection and transmission are determined by the refractive indices of the two media and the angle of incidence. In the common case of radiation in air striking the surface of a medium with refractive index n at normal incidence the reflection is given by (n-1)2/(n+1)2. So for a material with a refractive index of 1.5 the reflection at the surface is 4%. At other angles of incidence the reflection depends on the polarisation of the radiation.
19. The situation becomes more complicated, but also more interesting, when the second medium is absorbing. The refractive index is closely related to the absorption. Because the amount of reflection is determined by the refractive index the reflection changes wherever there is an absorption band. For an isolated absorption band the refractive index has a minimum on the high wavenumber side of the band and a maximum on the low wavenumber side. The reflection spectrum therefore resembles a first derivative, with a minimum to high wavenumber and a maximum to low wavenumber of the band centre. This can be seen in the spectra obtained by reflection from the surface of a block of polymethyl methacrylate and by transmission through a film of the same material in Fig.2.
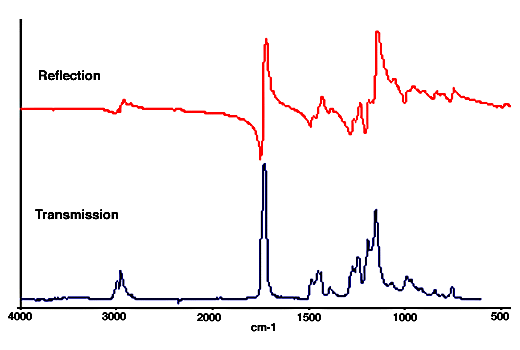
Figure 2. Reflection and transmission spectra of polymethylmethacrylate
20. The absorption spectrum can be calculated from the measured reflection spectrum by a mathematical operation called the Kramers-Kronig transformation. This is provided in most data manipulation packages used with FTIR spectrometers. Below is a comparison between the absorption spectrum of polymethylmethacrylate obtained by Kramers-Kronig transformation of the reflection spectrum and the spectrum of a thin film.
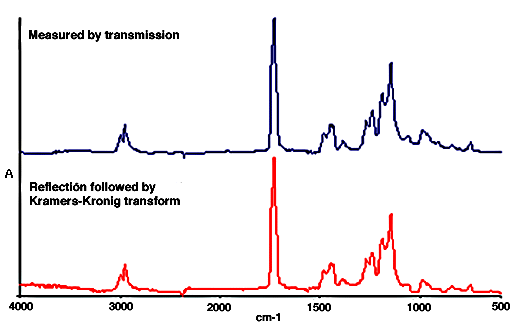
Figure 3. Transmission and Kramers-Kronig transformation of the reflection spectrum of polymethylmethacrylate
21. The previous examples consist of reflection from the front surface of a sample, sometimes called surface or Fresnel reflection. In many situations, for example reflection from a powder, the reflection spectra do not come from the front surface alone. Radiation that penetrates into the material can reappear after scattering or reflection at a second surface. When this radiation emerges it will have experienced some absorption, depending on the path traversed. Its contribution to the spectrum will have the general character of a transmission spectrum. Spectra of this type are called diffuse reflection spectra.
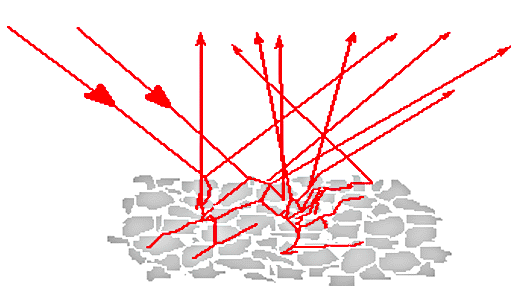
Figure 4. Diffuse reflection by a powder
22. The appearance of the spectra depends on the relative amounts of surface reflection and of radiation that has penetrated the sample. The main factors that influence this are the particle size and the strength of the absorptions. As the particle size increases the amount of radiation reappearing from within the sample is reduced because more is absorbed before it can be scattered or reflected back to the surface. This can be seen in the spectra of glycine below. It is clear that the derivative-like features of surface reflection are more prominent in the spectrum of the coarse powder. The spectrum of the finely ground material does show more features resembling absorption bands, but still is very different from a transmission spectrum.
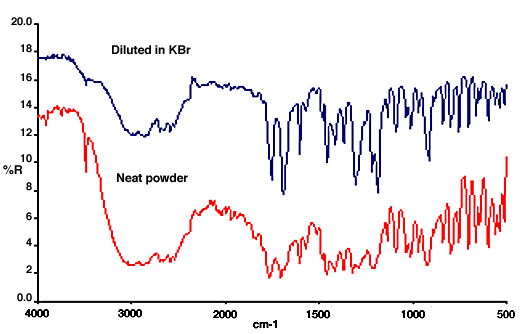
Figure 5. Diffuse reflection spectra of glycine (the upper one is offset for clarity).
23. The Kramers-Kronig transform is useful only for pure surface reflection. Spectra with a mixed character such as those above are rather intractable because the shapes and positions of the absorption bands cannot be identified. For qualitative identification it is necessary to maximise the contribution from radiation that has penetrated the sample. This can be done in the mid IR by reducing the particle size, ideally to less than 10mm, and diluting the sample in a non-absorbing matrix such as KBr. The effect of this is to reduce the amount of radiation that is absorbed internally before it can return to the surface. The resultant spectrum resembles a transmission spectrum except that there is a range of different pathlengths within the sample. In consequence the intensities of weaker bands appear enhanced relative to those of stronger bands. This distortion of the band intensities is minimised by ensuring that all the absorptions are weak.
24. Figure 6 shows diffuse reflection spectra of aspirin as a neat powder and diluted in KBr. The sample in KBr is very similar to the spectrum of a KBr disk. In the spectrum of the neat powder the stronger bands all have approximately the same intensities and their shapes are distorted by the contribution of surface reflection. However the weaker bands appear similar in both spectra.

Figure 6. Diffuse reflection spectra of aspirin.
25. Diffuse reflection spectra are often presented in what is called the Kubelka-Munk format rather than in absorbance, or more correctly log(1/R). The Kubelka-Munk intensity K-M is related to the measured reflectance R by an equation of the form: K-M = (1-R)2/2R.
This is said to provide band intensities that are proportional to concentration, as absorbance is for spectra measured by transmission. However the Kubelka-Munk relation was derived for conditions that are not usually met in mid-IR measurements and its use owes more to convention than to any practical advantage. This is clear from the fact that near IR diffuse reflection spectra are very widely used for quantitative analysis, but almost always in the log(1/R) format. A comparison between Kubelka-Munk and log(1/R) presentations is shown in Fig.7.
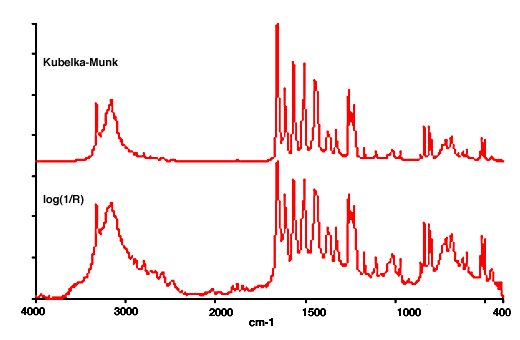
Figure 7. Diffuse reflection spectra of paracetamol in Kubelka-Munk and log(1/R) formats
26.Practical reflection measurements
Specular reflection
Reflection from a single surface is called specular reflection, as if from a mirror. Accessories for measuring specular reflection can be very simple, for example just involving two flat mirrors (Fig. 8). This type of accesssory would be suitable for measuring the reflection from a polished surface, such as the block of polymer used for the spectrum of Figure 1. For many samples the reflection spectrum is complicated by additional reflections from the back surface or by scattering within the sample. This kind of measurement is very successful for carbon-filled polymers with a suitably flat surface. The reason is that any light not reflected from the front surface is totally absorbed and so does not contribute to the spectrum.
Figure 8. Optical arrangement for measuring specular reflection.
27. This type of accessory can also be used to measure what is called a transmission-reflection (or transflectance) spectrum from thin coatings on metal surfaces. In this case the spectrum consists largely of radiation that passes through the coating and is reflected back from the metal surface. It resembles the spectrum that would be obtained from transmission through a film of the coating material. The spectrum will contain a contribution that is directly reflected from the front surface of the coating, but reflection from the metal surface is much higher. A typical spectrum from the coated inner surface of a soft drink can is seen in Fig.10. Coatings on non-metals are generally more difficult to examine. The spectra are not always dominated by the transmission-reflection component and absorption features of the substrate complicate the spectrum.
Figure 9. Transmission/reflection through a coating.

Figure 10. Transmission-reflection spectrum of drink can coating.
28. Transmission-reflection spectra are most useful for protective coatings and contaminants on metal surfaces. These are typically thicker than the wavelength of the infrared radiation being used. It is worth mentioning the rather different case of very thin layers on metal surfaces. Spectra of monomolecular layers can be measured fairly easily but special measurement conditions are used. These involve grazing incidence where the light path is almost parallel to the metal surface, because this greatly enhances the absorption intensity. The enhancement is caused by interaction between the electric field of the radiation and the conducting metal and extends only for a very short distance from the surface. Special grazing angle accessories are available for these measurements.
29.Diffuse Reflection
The radiation contributing to a diffuse reflection spectrum is typically spread over a range of angles and so cannot be collected efficiently with accessories designed to measure specular reflection. A typical arrangement for diffuse reflection measurements is shown below.
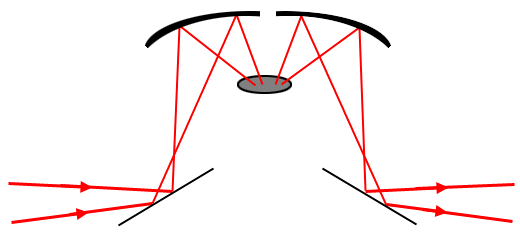
Figure 11. Optical arrangement for diffuse reflection.
30.Diffuse reflection spectra in the mid-IR are generally used for qualitative identification of powders. Because sample preparation need involve no more than mixing with KBr the method is simpler and more rapid than making a KBr disk. In principle diffuse reflection spectra can be used for quantitative analysis but this has proved much more successful with near IR data than in the mid-IR. Diffuse reflection can be a very convenient way of obtaining spectra from the surface of hard objects such as polymer mouldings. The method used is to rub the surface with abrasive paper so that a small amount is removed as a powder. The spectrum can be obtained simply by placing the abrasive paper in a diffuse reflection accessory. Figure 12 shows some typical spectra obtained from soft drink bottles obtained in this way.
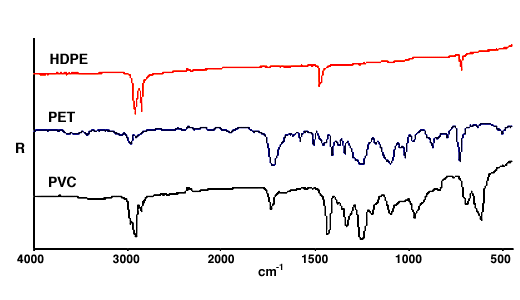
Figure 12. Spectra of soft drink bottles on silicon carbide abrasive.
REF: Int. J. Vib. Spect., [www.irdg.org/ijvs] 1, 5, 17-30 (1998)
Editor’ Note
Some aspects of this whole subject were covered in IJVS Volume 1, Edition 4, paragraphs 14-25 and 26-37.
