Molecular Vibrations - a simple tutorial - answers
Let's see how well you did...
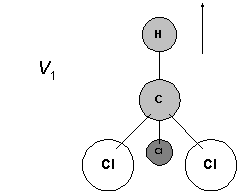
The CH bond is stretching. The H is so light and the CCl3 so massive the H does all the work. The rest hardly move at all.
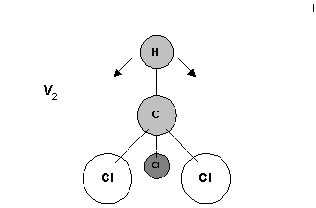
I've chosen the hydrogen deformation next. Again the H does all the work.
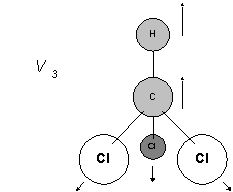
This is really the stretching of the C-Cl bonds all three in-phase with each other. The Cls are so heavy the C accompanied by its hydrogen leap up and down and the chlorines very sedately respond.
Let's see what happens if one C-Cl stretches while the others contract. Hard to see what the C and H do, so here goes.
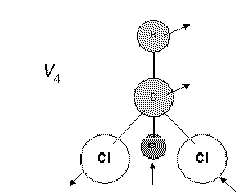
So V3 is the symmetrical C-Cl stretch and V4 its asymmetric partner.
Imagine an umbrella opening and closing except assume the canopy stays still and you leap up and down as you open and close it!
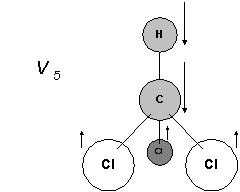
You would be very lucky to invent this one. The essential point is that the Cl / C \ Cl angle is opening and closing.
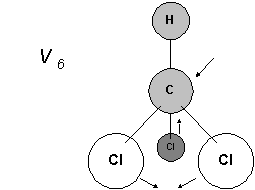
So V5 and V6 are related, V5 fully symmetrical V6 asymmetrical.
Degeneracy
Lets look at V4. We drew the left-hand chlorine moving out while the other two compressed. But why this particular chlorine?
So we have
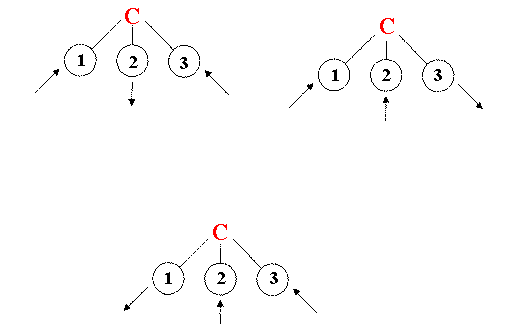
Three identical ways of drawing the same motion.
The identicality is called degeneracy by spectroscopists. Oddly, however the degeneracy is 2 not 3 because once you have drawn the first two diagrams the third is redundant. So V4 is doubly degenerate.
V6 behaves like V4 and so does V2. [Why you ask?]
The reason is that the H can wobble between different chlorines. Look down the CH bond from above
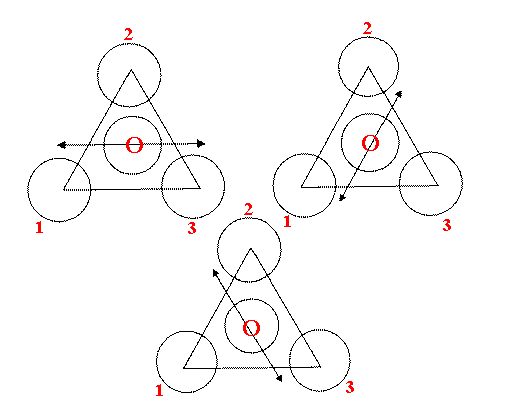
So we have
| V1 | V2 | V3 | V4 | V5 | V6 |
| x 2 | x 2 | x 2 | |||
| Total | 9 |
Assignment of the bands in the spectrum to fundamental modes
In Spectrum I, I show the Raman spectrum of liquid chloroform run at analytical resolution (4cm-1).
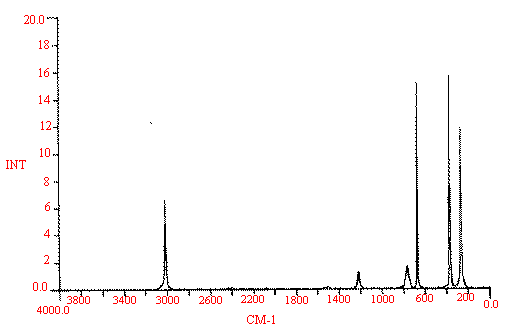
Now try an assignment yourself remembering the following rules:
For a given vibrator the stretching vibrations are typically twice that of the deformation or even greater.
Symmetrical vibrations tend to be intense in the Raman spectrum and weak in i.r. [If the molecule is centrosymmetric, the symmetric modes appear only in the Raman and vice-versa for the asymmetric ones - they occur only in the i.r.] and don't forget those group frequencies we all use.

