Preparation of Samples for IR Spectroscopy as KBr Disks
by Geoffrey Dent, ZENECA Specialties, P.O. Box 42, Hexagon House, Blackley, Manchester M9 8ZS
Powders, being examined by Infrared Spectroscopy, in transmission, are generally prepared by mulling in liquid paraffin (Nujol), or by grinding with potassium bromide (KBr) powder. The latter is then pressed into a disk. The method of preparation of a powder sample is generally determined by the information required or the chemical/physical stability of the sample. If information on the physical state, e.g. polymorphism, is required then grinding may change the state and mulling is preferable. Some substances, such as base hydrochloride, may exchange halogen with potassium bromide powder, again mulling is preferable. However most mulling agents contain bands in the spectrum which may mask bands in the sample spectrum. KBr does not contain bands in the mid-IR region of the spectrum, and therefore preparation as halide disks potentially loses less information. Samples dispersed in halide powder must be homogenously dispersed, with a particle size small enough not to cause scatter (theorectically < 2 microns). The strength of an IR absorption spectrum is dependant on the number of molecules in the beam. With a KBr disk the strength will be dependant on the amount and homogeneity of the sample dispersed in the KBr powder. The amounts stated below are for guidance only, the bulk density of the sample or other diluents may require these to be varied. They will also have to be varied according to the diameter of the disk required. The weights quoted are for a 16 mm diameter disk. Approximately half should be used for the 13 mm diameter disks.
Recommended Method
- Transfer weighed amounts of sample, approx 2 mg, and KBr powder, approx. 300 mg into an agate mortar. The KBr powder must be of spectroscopic grade purity, and be spectroscopically dry.
- Grind the powders together, with an agate pestle, until the sample is well dispersed and the mixture has the consistency of fine flour. With some very hard or crystalline powders this may not be possible by hand. If necessary, use mechanical or low temperature (liquid nitrogen cooled) grinding accessories.
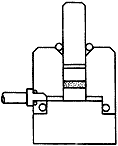
Figure 1: KBr die assembly.
- Assemble the die, with the lower pellet polished face up.
- Transfer the ground mixture into the cylinder bore so that it is evenly distributed across the polished face of the lower pellet. Gently inserting the plunger and lightly swivelling can often achieve a flat, even surface
- Insert the second pellet, polished face towards the mixture, into the bore followed by the plunger.
- Place the die assembly into a hydraulic press, between the ram and the piston.
- Ensure that the die is firmly held in the press.
- Connect a vacuum tube and switch on a High Vacuum pump.
- Leave the die assembly under vacuum for approximately 2 mins. This removes air from the disk.. (Some spectroscopists claim loose water is removed from the KBr and/or solvent from the sample. Others dispute this. Either way good vacuum leads to good disks)
- Increase pressure in the press to 15 tons (10 tons for 13 mm die, follow manufacturers instructions for max pressure with other diameter dies).
- After approximately 1 minute, slowly release the pressure.
- Carefully release the vacuum, and remove the die from the press.
- Dismantle the die, and transfer the KBr disk to a spectrometer disk holder. Avoid touching the faces of the disk.
- Check that the disk is translucent and that the sample is homogeneously distributed in the disk.
- Mount the disk holder in the spectrometer.
The spectrum quality is affected by the quality of the disk. The flatness of the baseline is dependent on the particle size and dispersion of the sample in the KBr powder. Check the disk and spectrum for the following faults :-
If the disk breaks on removal from the die, this indicates that the disk is too thin caused by too little powder, or too much pressure for too long. Remedy this effect by increasing the sample load. Also check that the correct pressure is used.
If the disk is not translucent, this can have numerous causes.
- Uneven distribution of powder in die
- Too much sample
- Too much KBr powder
- Poorly dispersed sample
- Water in disk
- Pressed at too low pressure or for too short a time
All except the last fault can be remedied by re-grinding and pressing with adjusted amounts. Water in the disk can be present due to a wet sample or wet KBr powder. Small amounts can be removed by the High Vacuum step (9). Heating the disk at approx 100°C for a few minutes and repressing the disk will sometimes remove residual water.
The disk turns brown. This could be due to the sample being an oxidising agent. Check the spectrum for halide degradation and re-examine as a mull if possible.
Truncated Bands. If the spectrum contains bands which have a flattened turning point and do not reach 0 % T, this is caused by a poorly dispersed sample or holes in the disk. Check the disk visually and if necessary repeat the preparation.
Sloping baseline. This is usually due to a poorly dispersed sample. Some substances are too hard (polymers) or too crystalline (e.g. Anthraquinone) to disperse properly. The latter can also cause bands to appear like a first derivative spectrum. This is due to refractive index changes and is known as the Christiansen Effect
The faults listed above have been commonly found, sometimes in publications, but is not necessarily exhaustive. Use of other halide salts can overcome some effects, or extend the range of the spectrum examined, e.g. CsI extends the lower wavenumber range from 400 to 200 cm -1. The use of these will be discussed in a later Edition. Poor sample preparation can lead to avoidable errors in interpretation of the resultant spectrum. A little care can avoid the need for repititous sample preparartion or embarrassing errors in results.
Spectra
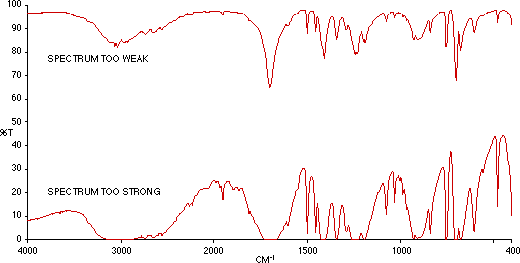
Figure 2: Phenyl Acetic Acid (i) Too Weak (ii) Too Strong.

Figure 3: Correctly Prepared KBr Disk of Phenyl Acetic Acid.
Editors Note: If you regularly use KBr disks, try Geoff’s instruction carefully and check that you can reproduce Fig 3, then use the method you normally use and compare. Might be quite a surprise. The background theory to the article is now covered by Bill Maddams.
REF: Int. J. Vib. Spect., [www.irdg.org/ijvs] 1, 1, 2 (1996)
