Investigation of drug/polymer interaction in glass solutions prepared by melt extrusion
Angus Forstera, b, John Hempenstallb, Thomas Radesa*
aSchool of Pharmacy,
University of Otago,
New Zealand
b GlaxoSmithKline, R&D,
Hertfordshire,
U.K.
* To whom correspondence should be addressed
Abstract
Indomethacin and nifedipine are poorly water-soluble drugs and exhibit dissolution rate limited oral bioavailability. In this study both drugs were prepared as glass solutions using melt extrusion in combination with the amorphous polymer polyvinylpyrrolidone (PVP). Attenuated total reflectance infrared spectroscopy (ATR), moisture analysis, X-ray powder diffraction, thermal analysis, scanning electron microscopy, dissolution and HPLC were used to examine the physico-chemical properties and chemical stability of the glass solutions. Both indomethacin and nifedipine glass solutions showed an increased rate of drug dissolution compared to crystalline drug and a physical mixture of crystalline drug and PVP. A marked physico-chemical interaction between the drug and polymer was found for both melt extrudates with ATR. However, this interaction was only found in amorphous drug/polymer physical mixtures with indomethacin and PVP. This interaction was probably caused by hydrogen bonding between the carbonyl group of the polymer and a H-donor group of the drug. Following storage for 4-12 weeks at 25°C/75 % relative humidity (RH) only indomethacin extrudates remained completely amorphous. The degree of H-bonding between drug and polymer may be an important factor in the stability of glass solutions.
Introduction
The successful formulation of poorly-water soluble drugs is one of the major problems in pharmaceutical manufacturing. Poorly water-soluble drugs, such as indomethacin and nifedipine, may show low and erratic oral bioavailability due to poor dissolution of the drug in the fluids of the gastrointestinal tract [1, 10].
The dissolution rate of a drug can be increased by several approaches including conversion of the crystalline drug into the amorphous state and by dispersion in a hydrophilic polymer [7, 12]. In addition, the presence of the polymer may increase the physical stability of the amorphous drug, which often limits application of the amorphous state in dosage form design. The amorphous state is characterised by its glass transition temperature (Tg) which refers to the point at which the supercooled melt freezes into a solid state known as a glass with increased viscosity compared to the ‘rubbery’ state at temperatures above the Tg. The physical stability of an amorphous substance is highest when stored as a glass, i.e. below the Tg. A glass solution is formed when drug and polymer are entirely miscible in the molten state and remain as an amorphous one-phase system when cooled.
Melt extrusion is a processing technique widely employed in the plastics industry and is currently being investigated for application in the pharmaceutical industry for the formation of drug / polymer solid dispersions [5, 2]. During melt extrusion drug is dispersed in the polymer by melting or plasticising both the drug and polymer using a combination of heat and mechanical energy supplied by either one or two rotating screws inside a heated barrel [2]. The material is then rapidly cooled. The process has been shown to be capable of producing glass solutions of drug and polymer [2].
The aim of this study was to prepare glass solutions of indomethacin and nifedipine in amorphous polymer using melt extrusion to increase drug dissolution and to determine the physico-chemical properties of the extrudate using a combination of techniques.
Materials and Methods
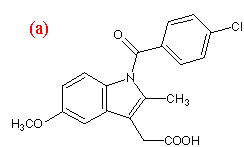
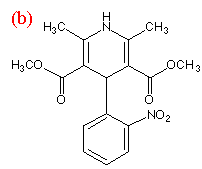
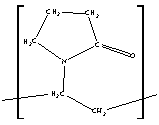
Indomethacin and nifedipine were purchased from Sigma Aldrich (Dorset, UK). Polyvinylpyrrolidone (PVP k30, average MW 50 000) was supplied by GlaxoWellcome (Ware, UK). The chemical structures of the compounds are shown in Figure 1. Nifedipine samples were protected from light at all times. All substances were used without further purification.
 (c)
(c)
Figure 1.Chemical structures of (a) indomethacin,
(b) nifedipine and (c) PVP
Melt extrusion
Melt extrusion was performed in a low humidity (< 25 % RH) and controlled temperature environment (20°C) using a Brabender Plasti-corder PL2000 extruder (Duisburg, Germany) with counter-rotating intermeshing twin screws. The extruder was kept at a constant temperature of 165°C for all drug/polymer blends. The powder blends were manually fed into the melt extruder. The screw speed was adjusted to 20-30 rpm, resulting in a residence time in the extruder of approximately 2-4 minutes. The melt extrudate was air-cooled on a conveyor belt and stored in sealed bags with desiccant (silica gel) at less than 25°C. Drug/polymer blends were extruded at a 1:1 mass ratio.
The melt extrudate was reduced in particle size by milling in a hammer mill through a 0.5 mm screen. A particle size range of 25 to 250 µm was selected for analysis.
Preparation of amorphous drugs
Amorphous drug samples were prepared by melting drug in stainless steel beakers. Melts were rapidly cooled by partially submerging the beaker into ice-cold water. The resulting mass was then pulverized by light grinding in a mortar. This method of preparing the amorphous state of the drugs has been used previously to produce amorphous indomethacin and partially amorphous nifedipine [4].
Preparation of physical mixtures
Drug/polymer physical mixtures were prepared by lightly grinding accurately weighed quantities of crystalline or amorphous drug and polymer in a mortar for 2 minutes at the required drug/polymer mass ratio. A particle size range of 25 to 250 µm was selected for analysis.
X-ray powder diffraction
X-ray powder diffraction (XRPD) was performed on a Philips X’pert MPD with a count time of 1 s and a step size of 0.04º 2q using Ni filtered Cu-ka radiation (40 kV and 50 mA). Samples were prepared by back-filling (300 mg).
Thermal Analysis
Samples were heated in a TA Instruments 2920 DSC (Surrey, UK) at 10 K min-1with a nitrogen purge at 20 ml min-1 using crimped aluminium pans with a pierced lid. Samples were run in duplicate and the sample size was 5-10 mg.
Modulated temperature DSC (MTDSC) was used to analyse the glass transition temperature (Tg) of the melt extrudates as the Tg was masked by a dehydration endotherm on analysis with conventional DSC. MTDSC was performed using the TA Instruments 2920 DSC with liquid nitrogen cooling accessory. Samples were sealed in aluminium pans and a sample size of 5-10 mg was used. A linear heating rate of 2 K min-1 with an oscillation of ± 0.25 K every 40 seconds was applied, ensuring at least six modulations per thermal event.
Infrared spectroscopy
IR spectroscopy is ideally suited to the investigation of the amorphous state as information on interactions on the molecular level can be derived. Recrystallisation of amorphous compounds has been reported when large levels of mechanical stress are applied and so preparation of samples for analysis should avoid excessive sample manipulation [8], such as used in the preparation of KBr discs and nujol mulls. ATR is an ideal approach to study amorphous materials by infrared spectroscopy as samples can be analysed without manipulation [3].
ATR was performed using a Nicolet Avatar FTIR spectrophotometer (Warwick, UK) and an Omni sampler ATR accessory (germanium crystal). A small amount of sample was placed on the crystal and constant pressure applied on the sample, ensuring good contact between the crystal and the sample. Background and sample measurements (64 scans, 4 wavenumber resolution) were performed for each sample. As PVP is a very hygroscopic material, samples containing PVP were dried prior to analysis, by either holding at 100°C in an oven for 3 hours or in the case of amorphous systems, storage for 48 hours under vacuum in a desiccator over silica gel. Spectra resulting from co-addition of spectra of individual components were calculated using Omnic (Nicolet) software.
Dissolution
Dissolution studies were undertaken using a USP II apparatus (37 ºC, 50 rpm) with UV analysis (Hewlett Packard 8453 diode array spectrophotometer).
Results and discussion
The extrudates were produced as thin spaghetti-like strands. No gross signs of decomposition were noted during melt extrusion and the drug content was 98.0 to 101.3 % of the expected value.
XRPD was used to determine the crystallinity of the melt extrudates following milling. The diffractograms from extrudates were compared to both crystalline drug and physical mixtures of drug and polymer. Figure 2 shows the X-ray diffractograms of nifedipine samples. Directly after preparation the melt extrudates exhibited a diffuse background XRPD pattern typical of amorphous compounds. Peaks associated with crystalline material were detectable in the diffractograms of crystalline drug and physical mixtures of crystalline drug and PVP.
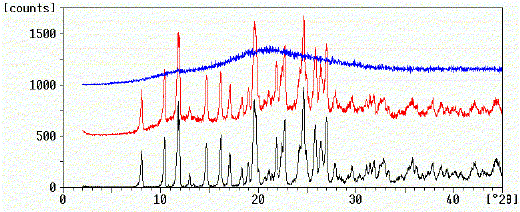
Figure 2. X-ray powder diffractograms of nifedipine.
black: Crystalline nifedipine
red: Crystalline nifedipine/PVP physical mixture
blue: Nifedipine/PVP melt extrudate
Using DSC and MTDSC all extrudates showed a single Tg at a temperature intermediate between the Tg of the individual components (Table 1). The Tg is an important indicator of the physical stability of the amorphous state and to ensure physical stability of the amorphous formulations over the product shelf-life it is recommended that they are stored well below the Tg (Yoshioka et al., 1995). However, the presence of moisture leads to a decrease in the Tg as water is a potent plasticiser (Hancock and Zografi, 1994).
The absence of detectable crystallinity with XRPD and DSC and the presence of a single Tg (MTDSC) are conclusive evidence of glass solution formation. In all cases, at the temperatures of extrusion used in this study chemical decomposition was less than 2 %. The drug dissolution from melt extrudates was significantly increased in comparison to both crystalline drug and drug/polymer physical mixtures (Table 1).
| Analysis | Indomethacin / PVPa | Nifedipine /PVP | ||||
| Initial | 8 weeks storage at | Initial | 8 weeks storage at | |||
| 25°C / 75 %RH |
25°C / <10 %RH |
25°C / 75 %RH |
25°C / <10 %RH |
|||
| Crystallinity (XRPD) |
Ab | A | A | A | C | A |
| Tg (°C) | 77.2 | 36.1 | 75.1 | 79.0 | 26.1 | 74.5 |
| % Water (w/w)c |
1.6 | 7.4 | 1.8 | 1.9 | 11.6 | 2.3 |
| Chemical degradationd |
<1% | – | – | 1.4% | – | – |
| Dissolution rate increasee |
2.4 /1.8 | – | – | 2.3 /1.4 | – | – |
| a Tg of PVP: 168 ° C b A = amorphous, C = crystalline peaks c Moisture content was determined in duplicate by Karl Fischer analysis (Turbo2 blending Karl Fischer, Orion, East Sussex, UK). d Chemical stability was determined with HPLC and online analysis was carried out at 210 nm and a drug specific wavelength (indomethacin 318 nm; nifedipine 340 nm). Solutions for analysis were injected in duplicate. Degradation is expressed as a percentage reduction in the drug peak. e Ratio of dissolution rate of the drug from the extrudate to dissolution rate of crystalline drug (first value) and ratio of dissolution rate of the drug from the extrudate to dissolution rate of the drug from a physical mixture of crystalline drug and PVP (second value). |
||||||
Table 1. Characterisation of melt extrudates after manufacture
and following 8 weeks storage
Infrared spectroscopy
Figure 3a shows the ATR spectra of various indomethacin samples and PVP over the spectral range 2000-700 cm-1. The spectra of crystalline drug, amorphous drug, PVP, physical mixtures and melt extrudates were collected. Co-addition spectra of crystalline or amorphous drug and polymer were calculated and compared to spectra of the physical mixtures and melt extrudates to detect the presence of interactions between the drug and polymer and are shown in Figure 3b.
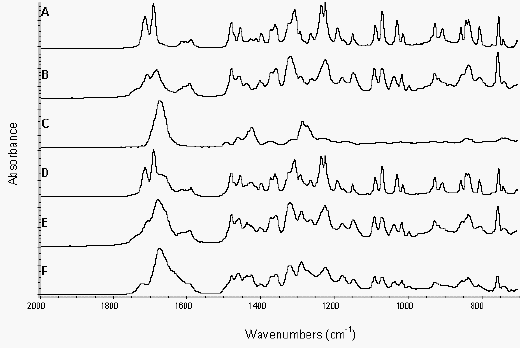
Figure 3a. ATR of indomethacin samples and PVP from 2000-700 cm-1
Crystalline drug (A), amorphous drug (B),
PVP (C), crystalline drug/PVP physical mixture (D),
amorphous drug/PVP physical mixture (E) and extrudate (F)
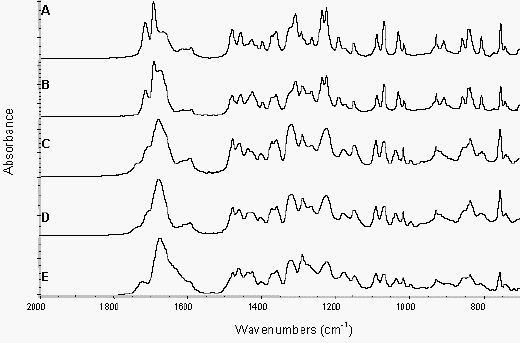
Figure 3b. ATR of indomethacin samples and
addition spectra from 2000-700 cm-1Crystalline drug/PVP physical mixture (A),
crystalline drug/PVP addition spectrum (B),
amorphous drug/PVP physical mixture (C),
amorphous drug/PVP addition spectrum (D) and extrudate (E)
The spectrum of amorphous drug was similar to that of crystalline indomethacin, with the amorphous spectrum being noticeably broader, due to the disorder inherent in the amorphous state. There were some differences in the spectra of amorphous and crystalline indomethacin, which could aid in further quantitative studies of the degree of crystallinity in indomethacin formulations (Figure 4). The ‘amorphous’ peak at 1437 cm-1 was masked by the presence of PVP and was, therefore, not detectable in the physical mixture or extrudate spectra (Figure 3a).
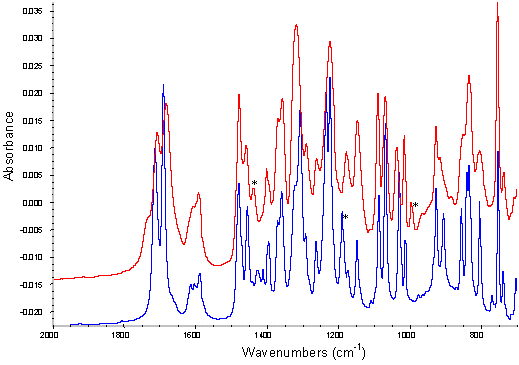
Figure 4. ATR of amorphous and crystalline indomethacin from 2000-700 cm-1
* Characteristic peaks at 995 and 1437 cm-1 for amorphous drug,
and 1189 cm-1 for crystalline drug
The physical mixtures of crystalline drug and polymer did not show any significant changes in the infrared spectra from that predicted by addition of the individual spectra of each component (Figure 3b). Comparison of the amorphous drug/PVP physical mixture or the extrudate sample to the addition spectra of amorphous drug and polymer however, exhibited changes in the region 1750-1600 cm-1 (Figure 3b). These differences can be seen more clearly in the spectra overlays shown in Figure 5, with the extrudate sample exhibiting a significant peak broadening in this region and the amorphous drug/PVP physical mixture a slight broadening. This region coincides with the indomethacin carboxylic stretch and the carbonyl stretch of the pyrrole ring of PVP (Figure 1) and the changes seen in the spectra are likely to reflect hydrogen bonding between these groups [13, 11]. PVP can only act as a hydrogen acceptor via the carbonyl or C-N groups in the pyrrole ring and previous work suggests that the access to the tertiary nitrogen is not favoured [11]. As shown in Figure 5, the interaction was more prominent in the melt extrudate with the carboxylic stretching region of indomethacin no longer being a discrete series of peaks. This interaction has been previously reported for solid dispersions of indomethacin/PVP [12]. Taylor and Zografi (1997), however, found no such interaction with simple physical mixing of amorphous indomethacin and PVP. Hydrogen bonding has been reported for PVP and ibuprofen, which is structurally related to indomethacin, following test tube mixing [11].
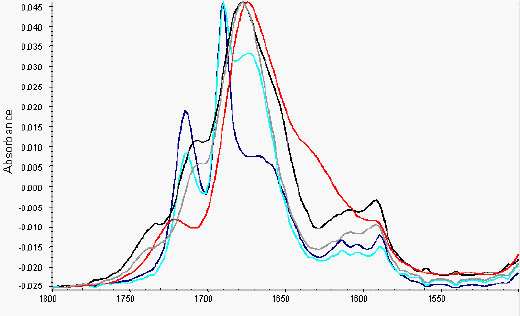
Figure 5. ATR of indomethacin samples (overlays) from 1800 – 1500 cm-1
red: Extrudate, blue: crystalline drug/PVP physical mixture,
turquoise(turquoise): crystalline drug/PVP addition,
black: amorphous drug/PVP PM,
grey: amorphous drug/PVP addition
The spectrum of amorphous nifedipine is less well defined than the respective crystalline spectra, with significant shifts and broadening of peaks noted in the amorphous samples (Figure 6). Figure 7a shows the ATR spectra of various nifedipine samples, over the range 4000 to 2500 cm-1 and Figure 7b is a comparison of the co-added spectra to the spectra of physical mixtures and extrudate. Spectra of physical mixtures of crystalline drug or amorphous drug with polymer are similar to those predicted by the addition of the spectra of the individual components (see Figure 8 for overlays). In comparison, the spectra of the melt extrudates shows a marked change in the region 3338 to 3322 cm-1, which reflects the weakening of the N – H stretching of the drug, probably due to H-bonding between the nifedipine and PVP via the secondary amine group of the drug (Figure 1). It should be noted from the ATR spectra that the intensity of the OH stretch due to absorbed water at around 3500 cm-1 for the PVP sample appears to be relatively weak (Figure 6a, C). This allows conclusions to be drawn with respect to interactions between the drug and polymer from the region of interest.
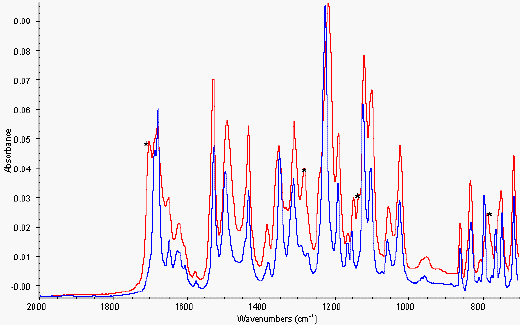
Figure 6. ATR of amorphous and crystalline nifedipine from 2000-700 cm-1
* Characteristic peaks at 785, 1147, 1281
and 1701 cm-1 for amorphous drug

Figure 7a. ATR of nifedipine samples and PVP from 3700-2500 cm-1
Crystalline drug (A), amorphous drug (B),
PVP (C), extrudate (D), amorphous drug/PVP physical mixture (E)
and crystalline drug/PVP physical mixture (F)
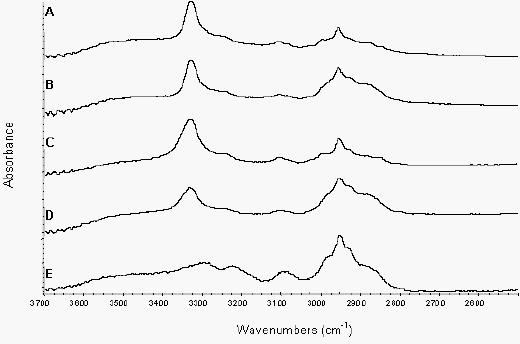
Figure 7b. ATR of nifedipine samples from 3700-2500 cm-1
Crystalline drug/PVP physical mixture (A),
crystalline drug/PVP addition spectrum (B),
amorphous drug/PVP physical mixture (C),
amorphous drug/PVP addition spectrum (D) and extrudate (E)
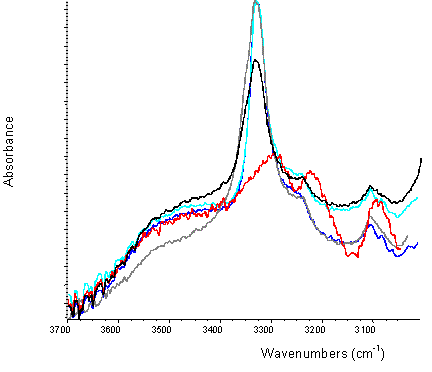
Figure 8. ATR of nifedipine samples (overlays) from 3700-3000 cm-1
red: Extrudate, blue: crystalline drug/PVP physical mixture,
turquoise (turquoise): crystalline drug/PVP addition,
black: amorphous drug/PVP PM,
grey: amorphous drug/PVP addition
Physical stability
Following eight weeks storage at 25°C/75% RH the Tg of nifedipine/PVP extrudate was 10°C below the Tg of indomethacin/PVP, despite the fact that the initial Tg values were almost identical (Table 1). The larger decrease in Tg upon storage was due to the higher water content of the nifedipine extrudate than that of the indomethacin extrudate at 25°C/75% RH (Table 1). The increased moisture content of nifedipine extrudate could be linked to the degree of interaction (i.e. H-bonding) between drug and polymer. Less H-bonding between drug and polymer could make more H-bonding sites available to water [9].
The results of a short-term stability study are shown in Table 1. Indomethacin/PVP remained amorphous when stored at 25°C/75% RH, whereas nifedipine extrudate showed the presence of peaks characteristic of crystalline nifedipine in the X-ray powder diffractogram. At 25°C/<10 % RH both samples remained amorphous.
Conclusions
Melt extrusion of indomethacin, and nifedipine with PVP resulted in the formation of glass solutions.
Both extrudates exhibited some degree of H-bonding with PVP detected using ATR. ATR was demonstrated to be a rapid means of characterising drug / excipient interactions and allowed highly hygroscopic samples to be analysed without significant sample preparation. Amorphous indomethacin also demonstrated H-bonding when physically mixed with PVP, but this was not evident with nifedipine. Indomethacin thus shows an increased tendency to form H-bonds with PVP and this may be related to the improved physical stability of the extrudate. However, from the presence of H-bonding indicated by IR spectroscopy alone, it should not be concluded that the stability of the glass solution is high, as demonstrated by the nifedipine extrudate. Physical stability of the melt extrudates was related to moisture content and Tg. It can be speculated that the degree of H-bonding between drug and polymer determines the moisture uptake by the sample and therefore, determines the physical stability.
Acknowledgements
The authors gratefully acknowledge the support of GlaxoSmithKline and the Vernon Tews Pharmacy Postgraduate Education Scholarship for AF.
References
- Ali, S.L. Nifedipine. Analytical profiles of drug substances. In Florey, K., ed. Academic Press, New York, (1989)
- Breitenbach, J., Berndl, G., Neumann, J., Rosenberg, J., Simon, D., Zeidler, J. Solid dispersions by an integrated melt extrusion system. Proc. Control. Rel. Soc. 804 – 805, (1998)
- Bugay, D.E. and Williams, A.C. Vibrational Spectroscopy. In Brittain, H.C., ed. Physical characterization of pharmaceutical solids. Marcel Dekker, New York, (1995)
- Forster, A.H., Hempenstall, J., Tucker, I. and Rades, T. Selection of drug candidates for melt extrusion by thermal methods. Proceed. Aust. Pharm. Sci. Assoc. 64, (1999)
- Gruenhagen, H.H. Polymer / Drug-melt extrusion: Therapeutic and Technological Appeal. Pharm. Technol. Eur. 11, 22 – 27, (1996)
- Hancock, B.C., Zografi, G. The relationship between the glass transition temperature and the water content of amorphous pharmaceutical solids. Pharm. Res. 11, 471 – 477, (1994)
- Hancock, B.C., Zografi, G. Characteristics and significance of the amorphous state in pharmaceutical systems. J. Pharm. Sci. 86, 1 – 12, (1997)
- Hirayama, F., Wang, Z. and Uekama, K. Effect of 2-hydroxypropyl-B-cyclodextran on crystallisation and polymorphic transition of nifedipine in solid state. Pharm. Res. 11, 1766 – 1770, (1994)
- Jans Frontini, H., Mielck, J.B. Stability of drugs in solid dispersions: effect of glass transition on degradation kinetics under stress in systems of reserpine and PVP. Eur. J. Pharm. Biopharm. 42, 303 – 312, 1996
- O’Brian M., McCauley, J., Cohen, E. Indomethacin. In Florey, K., ed. Analytical profiles of drug substances. Academic Press Inc. (1984)
- Sekizaki, H., Danjo, K., Eguchi, H., Yonezawa, Y., Sunada, H., Otsuka, A. Solid-state interaction of ibuprofen with polyvinylpyrrolidone. Chem. Pharm. Bull. 43, 988 – 993, (1995)
- Serajuddin, A.T.M. Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems and recent breakthroughs. J. Pharm. Sci. 88, 1058 – 1066, (1999)
- Taylor, L.S., Zografi, G. Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharm. Res. 14, 1691-1698, 1997
- Wade, A., Weller, P.J. Handbook of Pharmaceutical Excipients. American Pharmaceutical Association, Washington; Pharmaceutical Press, London, 1994
- Yoshioka, M., Hancock, B.C., Zografi, G. Inhibition of indomethacin crystallization in poly(vinylpyrrolidone) coprecipitates. J. Pharm. Sci. 84, 983 – 986, 1995
Received 19th October 2000, received in revised format 23rd February 2001,
accepted 24th February 2001.
REF: A.Forster, J. Hempenstall & T. Rades, Int. J. Vib. Spect., [www.irdg.org/ijvs] 5, 2, 6 (2001)